Activation of protein kinase C beta II by the stereo-specific phosphatidylserine receptor is required for phagocytosis of apoptotic thymocytes by resident murine tissue macrophages
- PMID: 12114511
- PMCID: PMC2640489
- DOI: 10.1074/jbc.M202967200
Activation of protein kinase C beta II by the stereo-specific phosphatidylserine receptor is required for phagocytosis of apoptotic thymocytes by resident murine tissue macrophages
Abstract
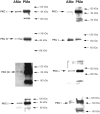
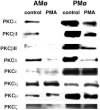
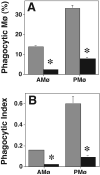
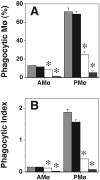
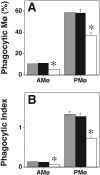
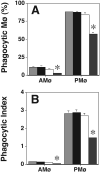
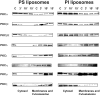
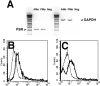
We showed previously that protein kinase C (PKC) is required for phagocytosis of apoptotic leukocytes by murine alveolar (AMø) and peritoneal macrophages (PMø) and that such phagocytosis is markedly lower in AMø compared with PMø. In this study, we examined the roles of individual PKC isoforms in phagocytosis of apoptotic thymocytes by these two Mø populations. By immunoblotting, AMø expressed equivalent PKC eta but lower amounts of other isoforms (alpha, betaI, betaII, delta, epsilon, mu, and zeta), with the greatest difference in betaII expression. A requirement for PKC betaII for phagocytosis was demonstrated collectively by phorbol 12-myristate 13-acetate-induced depletion of PKC betaII, by dose-response to PKC inhibitor Ro-32-0432, and by use of PKC betaII myristoylated peptide as a blocker. Exposure of PMø to phosphatidylserine (PS) liposomes specifically induced translocation of PKC betaII and other isoforms to membranes and cytoskeleton. Both AMø and PMø expressed functional PS receptor, blockade of which inhibited PKC betaII translocation. Our results indicate that murine tissue Mø require PKC betaII for phagocytosis of apoptotic cells, which differs from the PKC isoform requirement previously described in Mø phagocytosis of other particles, and imply that a crucial action of the PS receptor in this process is PKC betaII activation.
Figures


Similar articles
-
Distinct PKC isoforms mediate the activation of cPLA2 and adenylyl cyclase by phorbol ester in RAW264.7 macrophages.Br J Pharmacol. 1998 Dec;125(7):1601-9. doi: 10.1038/sj.bjp.0702219. Br J Pharmacol. 1998. PMID: 9884090 Free PMC article.
-
The receptor tyrosine kinase MerTK activates phospholipase C gamma2 during recognition of apoptotic thymocytes by murine macrophages.J Leukoc Biol. 2004 Apr;75(4):705-13. doi: 10.1189/jlb.0903439. Epub 2004 Jan 2. J Leukoc Biol. 2004. PMID: 14704368 Free PMC article.
-
Mechanism of inhibition of sequestration of protein kinase C alpha/betaII by ceramide. Roles of ceramide-activated protein phosphatases and phosphorylation/dephosphorylation of protein kinase C alpha/betaII on threonine 638/641.J Biol Chem. 2007 Jul 13;282(28):20647-56. doi: 10.1074/jbc.M609162200. Epub 2007 May 15. J Biol Chem. 2007. PMID: 17504762
-
Expression of protein kinase C-beta promotes the stimulatory effect of phorbol ester on phosphatidylethanolamine synthesis.Arch Biochem Biophys. 1997 Nov 1;347(1):37-44. doi: 10.1006/abbi.1997.0308. Arch Biochem Biophys. 1997. PMID: 9344462
-
Protein kinase C betaII activation induces angiotensin converting enzyme expression in neonatal rat cardiomyocytes.Cardiovasc Res. 2003 Jan;57(1):139-46. doi: 10.1016/s0008-6363(02)00610-7. Cardiovasc Res. 2003. PMID: 12504823
Cited by
-
Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase.Blood. 2005 Aug 1;106(3):1067-75. doi: 10.1182/blood-2004-08-3323. Epub 2005 Feb 17. Blood. 2005. PMID: 15718414 Free PMC article.
-
Protein kinase C-beta and -delta isoenzymes promote arachidonic acid production and proliferation of MonoMac-6 cells.J Mol Med (Berl). 2007 Sep;85(9):1031-42. doi: 10.1007/s00109-007-0209-y. Epub 2007 Jun 5. J Mol Med (Berl). 2007. PMID: 17549442
-
Resident murine alveolar and peritoneal macrophages differ in adhesion of apoptotic thymocytes.Am J Respir Cell Mol Biol. 2004 May;30(5):687-93. doi: 10.1165/rcmb.2003-0255OC. Epub 2003 Oct 3. Am J Respir Cell Mol Biol. 2004. PMID: 14527926 Free PMC article.
-
Tyro3 receptor tyrosine kinases in the heterogeneity of apoptotic cell uptake.Front Biosci (Landmark Ed). 2009 Jan 1;14(7):2631-46. doi: 10.2741/3401. Front Biosci (Landmark Ed). 2009. PMID: 19273223 Free PMC article. Review.
-
TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer.Adv Cancer Res. 2008;100:35-83. doi: 10.1016/S0065-230X(08)00002-X. Adv Cancer Res. 2008. PMID: 18620092 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

