Retrograde trans-synaptic transfer of green fluorescent protein allows the genetic mapping of neuronal circuits in transgenic mice
- PMID: 12114537
- PMCID: PMC126634
- DOI: 10.1073/pnas.152266799
Retrograde trans-synaptic transfer of green fluorescent protein allows the genetic mapping of neuronal circuits in transgenic mice
Abstract
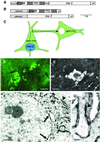
The function of the nervous system is a consequence of the intricate synaptic connectivity of its neurons. Our understanding of these highly complex networks has profited enormously from methods used over the past two decades that are based on the mechanical injection of tracer molecules into brain regions. We have developed a genetic system for the mapping of synaptic connections during development of the mammalian central nervous system and in the mature brain. It is based on the transsynaptic transfer of green fluorescent protein (GFP) in the brains of mice using a fusion protein with a nontoxic fragment of tetanus toxin (TTC) expressed in defined neurons. These transgenic mice allowed us to visualize neurons, at single-cell resolution, that are in synaptic contact by the detection of GFP in interconnected circuits. Targeted genetic expression with a specific promoter permitted us to transfer GFP to defined subsets of neurons and brain regions. GFP-TTC is coexpressed with a lacZ reporter gene to discriminate neurons that produce the tracer from cells that have acquired it transneuronally. The marker shows selective transfer in the retrograde direction. We have used electron microscopic detection of GFP to define the ultrastructural features of the system. Our work opens up a range of possibilities for brain slice and in vivo studies taking advantage of the fluorescence of GFP. We point the way toward the use of powerful multiphoton technology and set the stage for the transsynaptic transfer of other proteins in the brains of mice.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

