Monoallelic expression and methylation of imprinted genes in human and mouse embryonic germ cell lineages
- PMID: 12114541
- PMCID: PMC124986
- DOI: 10.1073/pnas.152327599
Monoallelic expression and methylation of imprinted genes in human and mouse embryonic germ cell lineages
Erratum in
- Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14255
Abstract
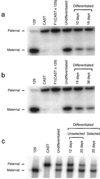
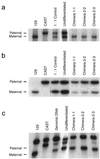
Imprinting is an epigenetic modification leading to monoallelic expression of some genes, and disrupted imprinting is believed to be a barrier to human stem cell transplantation, based on studies that suggest that epigenetic marks are unstable in mouse embryonic germ (EG) and embryonic stem (ES) cells. However, stem cell imprinting has not previously been examined directly in humans. We found that three imprinted genes, TSSC5, H19, and SNRPN, show monoallelic expression in in vitro differentiated human EG-derived cells, and a fourth gene, IGF2, shows partially relaxed imprinting at a ratio from 4:1 to 5:1, comparable to that found in normal somatic cells. In addition, we found normal methylation of an imprinting control region (ICR) that regulates H19 and IGF2 imprinting, suggesting that imprinting may not be a significant epigenetic barrier to human EG cell transplantation. Finally, we were able to construct an in vitro mouse model of genomic imprinting, by generating EG cells from 8.5-day embryos of an interspecific cross, in which undifferentiated cells show biallelic expression and acquire preferential parental allele expression after differentiation. This model should allow experimental manipulation of epigenetic modifications of cultured EG cells that may not be possible in human stem cell studies.
Figures

Comment in
-
Imprinted gene expression, transplantation medicine, and the "other" human embryonic stem cell.Proc Natl Acad Sci U S A. 2002 Aug 6;99(16):10243-5. doi: 10.1073/pnas.172384299. Epub 2002 Jul 30. Proc Natl Acad Sci U S A. 2002. PMID: 12149520 Free PMC article. Review. No abstract available.
References
-
- Lee J., Inoue, K., Ono, R., Ogonuki, N., Kohda, T., Kaneko-Ishino, T., Ogura, A. & Ishino, F. (2002) Development (Cambridge, U.K.) 129, 1807-1817. - PubMed
-
- Labosky P. A., Barlow, D. P. & Hogan, B. L. (1994) Development (Cambridge, U.K.) 120, 3197-3204. - PubMed
-
- Tada T., Tada, M., Hilton, K., Barton, S. C., Sado, T., Takagi, N. & Surani, M. A. (1998) Dev. Genes Evol. 207, 551-561. - PubMed
-
- Dean W., Bowden, L., Aitchison, A., Klose, J., Moore, T., Meneses, J. J., Reik, W. & Feil, R. (1998) Development (Cambridge, U.K.) 125, 2273-2282. - PubMed
-
- Humpherys D., Eggan, K., Akutsu, H., Hochedlinger, K., Rideout, W. M., 3rd, Biniszkiewicz, D., Yanagimachi, R. & Jaenisch, R. (2001) Science 293, 95-97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

