Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina
- PMID: 12114585
- PMCID: PMC166525
- DOI: 10.1104/pp.001909
Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina
Erratum in
- Plant Physiol. 2003 Jun;132(2):1115
Abstract
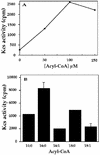
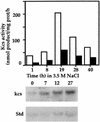
In studies of the outstanding salt tolerance of the unicellular green alga Dunaliella salina, we isolated a cDNA for a salt-inducible mRNA encoding a protein homologous to plant beta-ketoacyl-coenzyme A (CoA) synthases (Kcs). These microsomal enzymes catalyze the condensation of malonyl-CoA with acyl-CoA, the first and rate-limiting step in fatty acid elongation. Kcs activity, localized to a D. salina microsomal fraction, increased in cells transferred from 0.5 to 3.5 M NaCl, as did the level of the kcs mRNA. The function of the kcs gene product was directly demonstrated by the condensing activity exhibited by Escherichia coli cells expressing the kcs cDNA. The effect of salinity on kcs expression in D. salina suggested the possibility that salt adaptation entailed modifications in the fatty acid composition of algal membranes. Lipid analyses indicated that microsomes, but not plasma membranes or thylakoids, from cells grown in 3.5 M NaCl contained a considerably higher ratio of C18 (mostly unsaturated) to C16 (mostly saturated) fatty acids compared with cells grown in 0.5 M salt. Thus, the salt-inducible Kcs, jointly with fatty acid desaturases, may play a role in adapting intracellular membrane compartments to function in the high internal glycerol concentrations balancing the external osmotic pressure.
Figures



References
-
- Al-Hasan R, Ghannoum M, Sallal A, Abu-Elteen K, Radwan S. Correlative changes of growth, pigmentation and lipid composition of Dunaliella salina in response to halostress. J Gen Microbiol. 1987;133:2607–2616.
-
- Avron M. The osmotic components of halotolerant algae. Trends Biochem. 1986;11:5–6.
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

