Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses
- PMID: 12114590
- PMCID: PMC166530
- DOI: 10.1104/pp.001925
Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses
Abstract
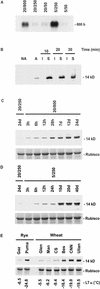
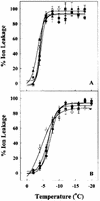
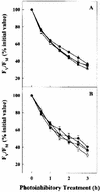
Cold acclimation and freezing tolerance are the result of complex interaction between low temperature, light, and photosystem II (PSII) excitation pressure. Previous results have shown that expression of the Wcs19 gene is correlated with PSII excitation pressure measured in vivo as the relative reduction state of PSII. Using cDNA library screening and data mining, we have identified three different groups of proteins, late embryogenesis abundant (LEA) 3-L1, LEA3-L2, and LEA3-L3, sharing identities with WCS19. These groups represent a new class of proteins in cereals related to group 3 LEA proteins. They share important characteristics such as a sorting signal that is predicted to target them to either the chloroplast or mitochondria and a C-terminal sequence that may be involved in oligomerization. The results of subcellular fractionation, immunolocalization by electron microscopy and the analyses of target sequences within the Wcs19 gene are consistent with the localization of WCS19 within the chloroplast stroma of wheat (Triticum aestivum) and rye (Secale cereale). Western analysis showed that the accumulation of chloroplastic LEA3-L2 proteins is correlated with the capacity of different wheat and rye cultivars to develop freezing tolerance. Arabidopsis was transformed with the Wcs19 gene and the transgenic plants showed a significant increase in their freezing tolerance. This increase was only evident in cold-acclimated plants. The putative function of this protein in the enhancement of freezing tolerance is discussed.
Figures






Similar articles
-
The interaction between cold and light controls the expression of the cold-regulated barley gene cor14b and the accumulation of the corresponding protein.Plant Physiol. 1999 Feb;119(2):671-80. doi: 10.1104/pp.119.2.671. Plant Physiol. 1999. PMID: 9952464 Free PMC article.
-
Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species.Plant Physiol. 2001 Nov;127(3):910-7. Plant Physiol. 2001. PMID: 11706173 Free PMC article.
-
Light-quality and temperature-dependent CBF14 gene expression modulates freezing tolerance in cereals.J Exp Bot. 2016 Mar;67(5):1285-95. doi: 10.1093/jxb/erv526. Epub 2015 Dec 27. J Exp Bot. 2016. PMID: 26712822
-
Harden the chloroplast to protect the plant.Physiol Plant. 2013 Jan;147(1):55-63. doi: 10.1111/j.1399-3054.2012.01689.x. Epub 2012 Oct 1. Physiol Plant. 2013. PMID: 22938043 Review.
-
Molecular characterization and functional analysis of elite genes in wheat and its related species.J Genet. 2010 Dec;89(4):539-54. doi: 10.1007/s12041-010-0074-2. J Genet. 2010. PMID: 21273706 Review.
Cited by
-
Genome-wide identification and expression analysis of late embryogenesis abundant protein-encoding genes in rye (Secale cereale L.).PLoS One. 2021 Apr 8;16(4):e0249757. doi: 10.1371/journal.pone.0249757. eCollection 2021. PLoS One. 2021. PMID: 33831102 Free PMC article.
-
Low-temperature exposure has immediate and lasting effects on the stress tolerance, chemotaxis and proteome of entomopathogenic nematodes.Parasitology. 2023 Jan;150(1):15-28. doi: 10.1017/S0031182022001445. Epub 2022 Oct 14. Parasitology. 2023. PMID: 36328953 Free PMC article.
-
Cbf genes of the Fr-A2 allele are differentially regulated between long-term cold acclimated crown tissue of freeze-resistant and - susceptible, winter wheat mutant lines.BMC Plant Biol. 2009 Mar 23;9:34. doi: 10.1186/1471-2229-9-34. BMC Plant Biol. 2009. PMID: 19309505 Free PMC article.
-
Wheat EST resources for functional genomics of abiotic stress.BMC Genomics. 2006 Jun 13;7:149. doi: 10.1186/1471-2164-7-149. BMC Genomics. 2006. PMID: 16772040 Free PMC article.
-
Overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis.Plant Mol Biol. 2004 Mar;54(5):743-53. doi: 10.1023/B:PLAN.0000040903.66496.a4. Plant Mol Biol. 2004. PMID: 15356392
References
-
- Akashi K, Grandjean O, Small I. Potential dual targeting of an Arabidopsisarchaebacterial-like histidyl-tRNA synthetase to mitochondria and chloroplast. FEBS Lett. 1998;431:39–44. - PubMed
-
- Asada K. Production and action of active oxygen species in photosynthesis tissues. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. FL: CRC Press Boca Raton; 1994. pp. 77–104.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

