Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum
- PMID: 12114593
- PMCID: PMC166533
- DOI: 10.1104/pp.002014
Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum
Abstract
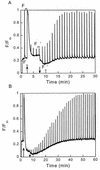
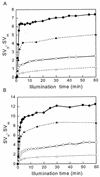
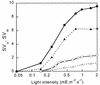
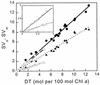
The pool size of the xanthophyll cycle pigment diadinoxanthin (DD) in the diatom Phaeodactylum tricornutum depends on illumination conditions during culture. Intermittent light caused a doubling of the DD pool without significant change in other pigment contents and photosynthetic parameters, including the photosystem II (PSII) antenna size. On exposure to high-light intensity, extensive de-epoxidation of DD to diatoxanthin (DT) rapidly caused a very strong quenching of the maximum chlorophyll fluorescence yield (F(m), PSII reaction centers closed), which was fully reversed in the dark. The non-photochemical quenching of the minimum fluorescence yield (F(o), PSII centers open) decreased the quantum efficiency of PSII proportionally. For both F(m) and F(o), the non-photochemical quenching expressed as F/F' - 1 (with F' the quenched level) was proportional to the DT concentration. However, the quenching of F(o) relative to that of F(m) was much stronger than random quenching in a homogeneous antenna could explain, showing that the rate of photochemical excitation trapping was limited by energy transfer to the reaction center rather than by charge separation. The cells can increase not only the amount of DT they can produce, but also its efficiency in competing with the PSII reaction center for excitation. The combined effect allowed intermittent light grown cells to down-regulate PSII by 90% and virtually eliminated photoinhibition by saturating light. The unusually rapid and effective photoprotection by the xanthophyll cycle in diatoms may help to explain their dominance in turbulent waters.
Figures
Similar articles
-
Detachment of the fucoxanthin chlorophyll a/c binding protein (FCP) antenna is not involved in the acclimative regulation of photoprotection in the pennate diatom Phaeodactylum tricornutum.Biochim Biophys Acta Bioenerg. 2017 Mar;1858(3):218-230. doi: 10.1016/j.bbabio.2016.12.005. Epub 2016 Dec 16. Biochim Biophys Acta Bioenerg. 2017. PMID: 27989819
-
The diatom Phaeodactylum tricornutum adjusts nonphotochemical fluorescence quenching capacity in response to dynamic light via fine-tuned Lhcx and xanthophyll cycle pigment synthesis.New Phytol. 2017 Apr;214(1):205-218. doi: 10.1111/nph.14337. Epub 2016 Nov 21. New Phytol. 2017. PMID: 27870063
-
Response of the diatom Phaeodactylum tricornutum to photooxidative stress resulting from high light exposure.PLoS One. 2012;7(6):e38162. doi: 10.1371/journal.pone.0038162. Epub 2012 Jun 1. PLoS One. 2012. PMID: 22675519 Free PMC article.
-
Structural features of the diatom photosystem II-light-harvesting antenna complex.FEBS J. 2020 Jun;287(11):2191-2200. doi: 10.1111/febs.15183. Epub 2020 Jan 7. FEBS J. 2020. PMID: 31854056 Review.
-
Alteration of photosystem II properties with non-photochemical excitation quenching.Philos Trans R Soc Lond B Biol Sci. 2000 Oct 29;355(1402):1405-18. doi: 10.1098/rstb.2000.0702. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11127995 Free PMC article. Review.
Cited by
-
Lighting the way: Compelling open questions in photosynthesis research.Plant Cell. 2024 Oct 3;36(10):3914-3943. doi: 10.1093/plcell/koae203. Plant Cell. 2024. PMID: 39038210 Free PMC article.
-
Comparison of photosynthetic responses between haptophyte Phaeocystis globosa and diatom Skeletonema costatum under phosphorus limitation.Front Microbiol. 2023 Jan 23;14:1085176. doi: 10.3389/fmicb.2023.1085176. eCollection 2023. Front Microbiol. 2023. PMID: 36756351 Free PMC article.
-
Carotenoid biosynthesis in diatoms.Photosynth Res. 2010 Nov;106(1-2):89-102. doi: 10.1007/s11120-010-9589-x. Epub 2010 Aug 24. Photosynth Res. 2010. PMID: 20734232 Review.
-
Impaired photoprotection in Phaeodactylum tricornutum KEA3 mutants reveals the proton regulatory circuit of diatoms light acclimation.New Phytol. 2022 Apr;234(2):578-591. doi: 10.1111/nph.18003. Epub 2022 Feb 21. New Phytol. 2022. PMID: 35092009 Free PMC article.
-
The central carbon and energy metabolism of marine diatoms.Metabolites. 2013 May 7;3(2):325-46. doi: 10.3390/metabo3020325. Metabolites. 2013. PMID: 24957995 Free PMC article.
References
-
- Anderson JM, Chow WS, Park Y-I. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res. 1995;46:129–139. - PubMed
-
- Arsalane W, Paresys G, Duval J-C, Wilhelm C, Conrad R, Büchel C. A new fluorometric device to measure the in vivo chlorophyll a fluorescence yield in microalgae and its use as a herbicide monitor. Eur J Phycol. 1993;28:247–252.
-
- Arsalane W, Rousseau B, Duval J-C. Influence of the pool size of the xanthophyll cycle on the effects of light stress in a diatom: competition between photoprotection and photoinhibition. Photochem Photobiol. 1994;60:237–243.
-
- Bassi R, Caffarri S. LHC proteins and the regulation of photosynthetic light harvesting function by xanthophylls. Photosynth Res. 2000;64:243–256. - PubMed
-
- Berkaloff C, Caron L, Rousseau B. Subunit organization of PSI particles from brown algae and diatoms: polypeptide and pigments analysis. Photosynth Res. 1990;23:181–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

