Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine
- PMID: 12117872
- PMCID: PMC1773316
- DOI: 10.1136/gut.51.2.155
Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine
Abstract
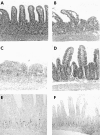
Background and aims: Mucosal flattening and epithelial cell apoptosis are typical features of T cell induced inflammatory diseases of the bowel, such as coeliac disease and graft versus host disease. Mice injected with a T cell activating anti-CD3 antibody develop a severe diarrhoeal illness. We describe the histological features of this enteropathy and define the effector mechanisms involved in T cell induced mucosal injury in this in vivo model.
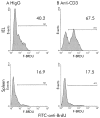
Methods: Wild-type and genetically modified mice were injected with the anti-CD3 antibody 3C11 (50 microg). Changes in the murine intestine were characterised by light microscopy analysis and terminal uridine nick-end labelling (TUNEL) assay. The role of perforin, Fas/Fas ligand (FasL), tumour necrosis factor alpha (TNF-alpha), and interferon gamma (IFN-gamma) in T cell induced mucosal damage was assessed using selected immunodeficient mouse strains.
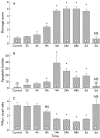
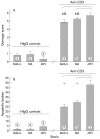
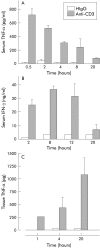
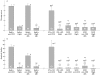
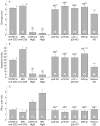
Results: T cell activation caused severe damage, including small intestinal mucosal flattening and apoptosis of crypt epithelial cells. Mucosal damage was unaltered in anti-CD3 treated mice lacking IFN-gamma, Fas, or TNF-alpha receptors. In mice lacking TNF-alpha receptors and Fas (TNF-R1xR2 lpr/lpr strain), enterocyte apoptosis was diminished but there was no significant reduction in tissue damage. Apoptosis and mucosal injury were significantly reduced in perforin knockout mice. Abrogation of both FasL and perforin (perforin KOxgld mice) further significantly reduced tissue damage and apoptotic bodies.
Conclusions: T cell induced mucosal injury is mediated by the combined effect of multiple pathways but predominantly by perforin. The redundancy of the mechanisms of tissue damage will have significant impact on therapeutic strategies aimed at specific and targeted inhibition of inflammatory processes.
Figures
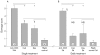
References
-
- Strater J, Wellisch I, Riedl S, et al. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology 1997;113:16. - PubMed
-
- Yang X, Stennicke HR, Wang B, et al. Granzyme B mimics apical caspases. Description of a unified pathway for trans-activation of executioner caspase-3 and -7. J Biol Chem 1998;273:34278–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
