Phosphoantigen presentation by macrophages to mycobacterium tuberculosis--reactive Vgamma9Vdelta2+ T cells: modulation by chloroquine
- PMID: 12117907
- PMCID: PMC128132
- DOI: 10.1128/IAI.70.8.4019-4027.2002
Phosphoantigen presentation by macrophages to mycobacterium tuberculosis--reactive Vgamma9Vdelta2+ T cells: modulation by chloroquine
Abstract
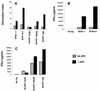
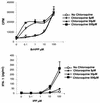
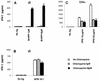
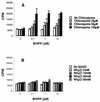
Vgamma9Vdelta2+ T cells (gammadelta T cells) are activated by Mycobacterium tuberculosis and recognize mycobacterial nonpeptide phosphoantigens. The role of antigen-presenting cells in the processing and presentation of phosphoantigens to Vgamma9Vdelta2+ T cells is not understood. We analyzed the role of macrophages for activation of gammadelta T cells by a new synthetic phosphoantigen bromohydrin pyrophosphate (BrHPP) and M. tuberculosis. Macrophages greatly increased gammadelta T-cell activation by both BrHPP and M. tuberculosis. Fixation of macrophages before infection demonstrated that uptake of M. tuberculosis was required for presentation to gammadelta T cells. Antigens of M. tuberculosis remained stably associated with macrophage surface and were not removed by paraformaldehyde fixation or washing. Macrophages processed M. tuberculosis for gammadelta T cells through a brefeldin A-insensitive pathway, suggesting that transport through the endoplasmic reticulum and Golgi complex of a putative presenting molecule is not important in the early processing of M. tuberculosis antigens for gammadelta T cells. Processing of M. tuberculosis was not eliminated by chloroquine, indicating that processing of gammadelta antigens is not dependent on acidic pH in the lysosomes. Chloroquine treatment of BrHPP-pulsed macrophages increased activation of gammadelta T cells. Ammonium chloride treatment of macrophages did not increase reactivity of gammadelta T cells to BrHPP, indicating that the effect of chloroquine was independent of pH changes in endosomes. Chloroquine, by inhibiting membrane traffic, may increase association and retention of phosphoantigens with cell surface membrane molecules on macrophages.
Figures




Similar articles
-
Processing of Mycobacterium tuberculosis bacilli by human monocytes for CD4+ alphabeta and gammadelta T cells: role of particulate antigen.Infect Immun. 1998 Jan;66(1):98-106. doi: 10.1128/IAI.66.1.98-106.1998. Infect Immun. 1998. PMID: 9423845 Free PMC article.
-
In vivo gammadelta T cell priming to mycobacterial antigens by primary Mycobacterium tuberculosis infection and exposure to nonpeptidic ligands.Mol Med. 1999 Jul;5(7):471-6. Mol Med. 1999. PMID: 10449808 Free PMC article.
-
In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model.J Immunol. 2005 Oct 15;175(8):5471-80. doi: 10.4049/jimmunol.175.8.5471. J Immunol. 2005. PMID: 16210655
-
Gammadelta T cells and Mycobacterium tuberculosis.Microbes Infect. 1999 Mar;1(3):187-95. doi: 10.1016/s1286-4579(99)80033-1. Microbes Infect. 1999. PMID: 10801229 Review.
-
Multifunctional immune responses of HMBPP-specific Vγ2Vδ2 T cells in M. tuberculosis and other infections.Cell Mol Immunol. 2013 Jan;10(1):58-64. doi: 10.1038/cmi.2012.46. Epub 2012 Nov 12. Cell Mol Immunol. 2013. PMID: 23147720 Free PMC article. Review.
Cited by
-
γδ T cell receptor ligands and modes of antigen recognition.Arch Immunol Ther Exp (Warsz). 2011 Apr;59(2):117-37. doi: 10.1007/s00005-011-0118-1. Epub 2011 Feb 6. Arch Immunol Ther Exp (Warsz). 2011. PMID: 21298486 Free PMC article. Review.
-
CD4+ CD25(high) Foxp3+ regulatory T cells downregulate human Vdelta2+ T-lymphocyte function triggered by anti-CD3 or phosphoantigen.Immunology. 2009 Jul;127(3):398-407. doi: 10.1111/j.1365-2567.2008.02982.x. Epub 2008 Nov 14. Immunology. 2009. PMID: 19019089 Free PMC article.
-
Sensing of Pyrophosphate Metabolites by Vγ9Vδ2 T Cells.Front Immunol. 2015 Jan 22;5:688. doi: 10.3389/fimmu.2014.00688. eCollection 2014. Front Immunol. 2015. PMID: 25657647 Free PMC article. Review.
-
Non-structure protein ORF1ab (NSP8) in SARS-CoV-2 contains potential γδT cell epitopes.Front Microbiol. 2022 Jul 22;13:936272. doi: 10.3389/fmicb.2022.936272. eCollection 2022. Front Microbiol. 2022. PMID: 35935236 Free PMC article.
-
Human αβ and γδ T Cells in Skin Immunity and Disease.Front Immunol. 2018 Jun 6;9:1304. doi: 10.3389/fimmu.2018.01304. eCollection 2018. Front Immunol. 2018. PMID: 29928283 Free PMC article. Review.
References
-
- Ab, B. K., R. Kiessling, J. D. Van Embden, J. E. Thole, D. S. Kumararatne, P. Pisa, A. Wondimu, and T. H. Ottenhoff. 1990. Induction of antigen-specific CD4+ HLA-DR-restricted cytotoxic T lymphocytes as well as nonspecific nonrestricted killer cells by the recombinant mycobacterial 65-kDa heat-shock protein. Eur. J. Immunol. 20:369-377. - PubMed
-
- Adorini, L., S. J. Ullrich, E. Appella, and S. Fuchs. 1990. Inhibition by brefeldin A of presentation of exogenous protein antigens to MHC class II-restricted T cells. Nature 346:63-66. - PubMed
-
- Allison, T. J., C. C. Winter, J. J. Fournie, M. Bonneville, and D. N. Garboczi. 2001. Structure of a human γδ T-cell antigen receptor. Nature 411:820-824. - PubMed
-
- Balaji, K. N., S. K. Schwander, E. A. Rich, and W. H. Boom. 1995. Alveolar macrophages as accessory cells for human γδ T cells activated by Mycobacterium tuberculosis. J. Immunol. 154:5959-5968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

