Eighty-kilodalton N-terminal moiety of Bordetella pertussis filamentous hemagglutinin: adherence, immunogenicity, and protective role
- PMID: 12117922
- PMCID: PMC128203
- DOI: 10.1128/IAI.70.8.4142-4147.2002
Eighty-kilodalton N-terminal moiety of Bordetella pertussis filamentous hemagglutinin: adherence, immunogenicity, and protective role
Abstract
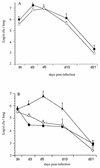
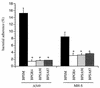
Bordetella pertussis, the etiological agent of whooping cough, produces a number of factors, such as toxins and adhesins, that are required for full expression of virulence. Filamentous hemagglutinin (FHA) is the major adhesin of B. pertussis. It is a protein of approximately 220 kDa, found both associated at the bacterial cell surface and secreted into the extracellular milieu. Despite its importance in B. pertussis pathogenesis and its inclusion in most acellular pertussis vaccines, little is known about the functional importance of individual domains in infection and in the induction of protective immunity. In this study, we analyzed the role of the approximately 80-kDa N-terminal domain of FHA, designated Fha44, in B. pertussis adherence, colonization, and immunogenicity. Although Fha44 contains the complete heparan sulfate-binding domain, it is not sufficient for adherence to epithelial cells or macrophages. It also cannot replace FHA during colonization of the mouse respiratory tract. Infection with a B. pertussis strain producing Fha44 instead of FHA does not induce anti-FHA antibodies, whereas such antibodies can readily be induced by intranasal administration of purified Fha44. In addition, mice immunized with purified Fha44 were protected against challenge with wild-type B. pertussis, indicating that Fha44 contains protective epitopes. Compared to FHA, Fha44 is much smaller and much more soluble and is therefore easier to purify and to store. These advantages may perhaps warrant considering Fha44 for inclusion in acellular pertussis vaccines.
Figures


References
-
- Cahill, E. S., D. T. O'Hagan, L. Illum, A. Barnard, K. H. G. Mills, and K. Redhead. 1995. Immune responses and protection against Bordetella pertussis infection after intranasal immunization of mice with filamentous haemagglutinin in solution or incorporated in biodegradable microparticles. Vaccine 13:455-462. - PubMed
-
- Cahill, E. S., D. T. O'Hagan, L. Illum, and K. Redhead. 1993. Mice are protected against Bordetella pertussis infection by intra-nasal immunization with filamentous haemagglutinin. FEMS Microbiol. Lett. 107:211-216. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

