Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis
- PMID: 12117930
- PMCID: PMC128156
- DOI: 10.1128/IAI.70.8.4215-4225.2002
Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis
Abstract
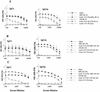
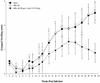
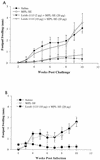
Development of an effective vaccine against Leishmania infection is a priority of tropical disease research. We have recently demonstrated protection against Leishmania major in the murine and nonhuman primate models with individual or combinations of purified leishmanial recombinant antigens delivered as plasmid DNA constructs or formulated with recombinant interleukin-12 (IL-12) as adjuvant. In the present study, we immunized BALB/c mice with a recombinant polyprotein comprising a tandem fusion of the leishmanial antigens thiol-specific antioxidant, L. major stress-inducible protein 1 (LmSTI1), and Leishmania elongation initiation factor (LeIF) delivered with adjuvants suitable for human use. Aspects of the safety, immunogenicity, and vaccine efficacy of formulations with each individual component, as well as the polyprotein referred to as Leish-111f, were assessed by using the L. major challenge model with BALB/c mice. No adverse reactions were observed when three subcutaneous injections of the Leish-111f polyprotein formulated with either MPL-squalene (SE) or Ribi 529-SE were given to BALB/c mice. A predominant Th1 immune response characterized by in vitro lymphocyte proliferation, gamma interferon production, and immunoglobulin G2A antibodies was observed with little, if any, IL-4. Moreover, Leish-111f formulated with MPL-SE conferred immunity to leishmaniasis for at least 3 months. These data demonstrate success at designing and developing a prophylactic leishmaniasis vaccine that proved effective in a preclinical model using multiple leishmanial antigens produced as a single protein delivered with a powerful Th1 adjuvant suitable for human use.
Figures



Similar articles
-
Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells.Infect Immun. 2007 Sep;75(9):4648-54. doi: 10.1128/IAI.00394-07. Epub 2007 Jul 2. Infect Immun. 2007. PMID: 17606603 Free PMC article.
-
Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c mice.Infect Immun. 2002 Jun;70(6):2828-36. doi: 10.1128/IAI.70.6.2828-2836.2002. Infect Immun. 2002. PMID: 12010969 Free PMC article.
-
Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant.Vaccine. 2002 Sep 10;20(27-28):3292-303. doi: 10.1016/s0264-410x(02)00302-x. Vaccine. 2002. PMID: 12213399
-
Activation of skin dendritic cells by immunostimulatory DNA.Springer Semin Immunopathol. 2000;22(1-2):45-54. doi: 10.1007/s002810000027. Springer Semin Immunopathol. 2000. PMID: 10944799 Review. No abstract available.
-
Vaccination against cutaneous leishmaniasis: current status.Am J Clin Dermatol. 2002;3(8):557-70. doi: 10.2165/00128071-200203080-00006. Am J Clin Dermatol. 2002. PMID: 12358557 Review.
Cited by
-
Immunization with Leishmania major exogenous antigens protects susceptible BALB/c mice against challenge infection with L. major.Infect Immun. 2004 Oct;72(10):5654-61. doi: 10.1128/IAI.72.10.5654-5661.2004. Infect Immun. 2004. PMID: 15385463 Free PMC article.
-
An α-Gal-containing neoglycoprotein-based vaccine partially protects against murine cutaneous leishmaniasis caused by Leishmania major.PLoS Negl Trop Dis. 2017 Oct 25;11(10):e0006039. doi: 10.1371/journal.pntd.0006039. eCollection 2017 Oct. PLoS Negl Trop Dis. 2017. PMID: 29069089 Free PMC article.
-
In Commemoration of Dr. Farrokh Modabber: An Iranian Pioneer of Cellular Immunology, and Leishmaniases Vaccine Research in Iran and the World.Arch Iran Med. 2024 Sep 1;27(9):530-537. doi: 10.34172/aim.28959. Epub 2024 Sep 1. Arch Iran Med. 2024. PMID: 39465529 Free PMC article.
-
Targeting Leishmania major Antigens to Dendritic Cells In Vivo Induces Protective Immunity.PLoS One. 2013 Jun 26;8(6):e67453. doi: 10.1371/journal.pone.0067453. Print 2013. PLoS One. 2013. PMID: 23840706 Free PMC article.
-
Identification of Leishmania infantum chagasi proteins in urine of patients with visceral leishmaniasis: a promising antigen discovery approach of vaccine candidates.Parasite Immunol. 2012 Jul;34(7):360-71. doi: 10.1111/j.1365-3024.2012.01365.x. Parasite Immunol. 2012. PMID: 22443237 Free PMC article.
References
-
- Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257:545-548. - PubMed
-
- Berman, J. D. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684-703. - PubMed
-
- Campos-Neto, A., R. Porrozzi, K. Greeson, R. N. Coler, J. R. Webb, Y. A. W. Seiky, S. G. Reed, and G. Grimaldi, Jr. 2001. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect. Immun. 69:4103-4108. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

