Contribution of phospholipomannan to the surface expression of beta-1,2-oligomannosides in Candida albicans and its presence in cell wall extracts
- PMID: 12117941
- PMCID: PMC128193
- DOI: 10.1128/IAI.70.8.4323-4328.2002
Contribution of phospholipomannan to the surface expression of beta-1,2-oligomannosides in Candida albicans and its presence in cell wall extracts
Abstract
beta-1,2-Oligomannosides (beta-1,2-Man) derived from Candida albicans mannan have been shown to act as adhesins and to induce protective antibodies. We used monoclonal antibodies specific for beta-1,2-Man in electron, confocal, and fluorescence microscopy to study the surface expression of beta-1,2-Man epitopes. These monoclonal antibodies were also used for Western blotting of cell surface extracts to study the nature of the molecules expressing the beta-Man epitopes. Evidence was obtained for the contribution of a glycolipid, phospholipomannan (PLM), to the complex expression of beta-1,2-Man epitopes at the cell wall surfaces of yeasts grown on solid media. PLM was present in intercellular matrixes of colonies grown on agar and was detected as a contaminant in mannan batches prepared by conventional methods.
Figures
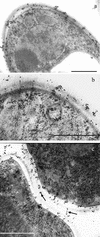

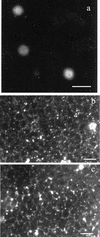
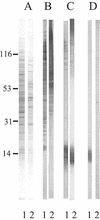
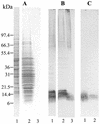

Similar articles
-
Evidence for different mannosylation processes involved in the association of beta-1,2-linked oligomannosidic epitopes in Candida albicans mannan and phospholipomannan.Microbiology (Reading). 1996 Aug;142 ( Pt 8):2263-70. doi: 10.1099/13500872-142-8-2263. Microbiology (Reading). 1996. PMID: 8760938
-
Candida albicans phospholipomannan, a new member of the fungal mannose inositol phosphoceramide family.J Biol Chem. 2002 Oct 4;277(40):37260-71. doi: 10.1074/jbc.M202295200. Epub 2002 Jul 22. J Biol Chem. 2002. PMID: 12138092
-
Early signal transduction induced by Candida albicans in macrophages through shedding of a glycolipid.J Infect Dis. 1998 Sep;178(3):792-802. doi: 10.1086/515361. J Infect Dis. 1998. PMID: 9728549
-
N-glycosylation of yeast, with emphasis on Candida albicans.Med Mycol. 2001;39 Suppl 1:75-86. Med Mycol. 2001. PMID: 11800271 Review.
-
Candida albicans phospholipomannan: a sweet spot for controlling host response/inflammation.Semin Immunopathol. 2015 Mar;37(2):123-30. doi: 10.1007/s00281-014-0461-5. Epub 2014 Nov 14. Semin Immunopathol. 2015. PMID: 25394861 Review.
Cited by
-
Deficient beta-mannosylation of Candida albicans phospholipomannan affects the proinflammatory response in macrophages.PLoS One. 2013 Dec 19;8(12):e84771. doi: 10.1371/journal.pone.0084771. eCollection 2013. PLoS One. 2013. PMID: 24367694 Free PMC article.
-
Advances in combating fungal diseases: vaccines on the threshold.Nat Rev Microbiol. 2007 Jan;5(1):13-28. doi: 10.1038/nrmicro1537. Epub 2006 Dec 11. Nat Rev Microbiol. 2007. PMID: 17160002 Free PMC article. Review.
-
Virulence of the fungal pathogen Candida albicans requires the five isoforms of protein mannosyltransferases.Infect Immun. 2005 Aug;73(8):4571-80. doi: 10.1128/IAI.73.8.4571-4580.2005. Infect Immun. 2005. PMID: 16040968 Free PMC article.
-
Antibodies as Models and Tools to Decipher Candida albicans Pathogenic Development: Review about a Unique Monoclonal Antibody Reacting with Immunomodulatory Adhesins.J Fungi (Basel). 2023 May 31;9(6):636. doi: 10.3390/jof9060636. J Fungi (Basel). 2023. PMID: 37367572 Free PMC article. Review.
-
Initiation of phospholipomannan β-1,2 mannosylation involves Bmts with redundant activity, influences its cell wall location and regulates β-glucans homeostasis but is dispensable for Candida albicans systemic infection.Biochimie. 2016 Jan;120:96-104. doi: 10.1016/j.biochi.2015.09.032. Epub 2015 Sep 30. Biochimie. 2016. PMID: 26427558 Free PMC article.
References
-
- Barnes, P. F., D. Chatterjee, J. S. Abrams, S. Lu, E. Wang, M. Yamamura, P. J. Brennan, and R. L. Modlin. 1992. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J. Immunol. 149:541-547. - PubMed
-
- Bobichon, H., D. Gache, and P. Bouchet. 1994. Ultrarapid cryofixation of Candida albicans: evidence for a fibrillar reticulated external layer and mannan channels within the cell wall. Cryo-Letters 15:161-172.
-
- Calera, J. A., and R. Calderone. 1999. Flocculation of hyphae is associated with a deletion in the putative CaHK1 two-component histidine kinase gene from Candida albicans. Microbiology 145:1431-1442. - PubMed
-
- Cantelli, C., P. A. Trinel, A. Bernigaud, T. Jouault, L. Polonelli, and D. Poulain. 1995. Mapping of β-1,2-linked oligomannosidic epitopes among glycoconjugates of Candida species. Microbiology 141:2693-2697. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

