Identification of chromosomal Shigella flexneri genes induced by the eukaryotic intracellular environment
- PMID: 12117948
- PMCID: PMC128171
- DOI: 10.1128/IAI.70.8.4379-4388.2002
Identification of chromosomal Shigella flexneri genes induced by the eukaryotic intracellular environment
Abstract
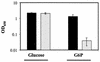
Upon entry into the eukaryotic cytosol, the facultative intracellular bacterium Shigella flexneri is exposed to an environment that may necessitate the expression of particular genes for it to survive and grow intracellularly. To identify genes that are induced in response to the intracellular environment, we screened a library containing fragments of the S. flexneri chromosome fused to a promoterless green fluorescent protein gene (gfp). Bacteria containing promoter fusions that had a higher level of gfp expression when S. flexneri was intracellular (in Henle cells) than when S. flexneri was extracellular (in Luria-Bertani broth) were isolated by using fluorescence-activated cell sorting. Nine different genes with increased expression in Henle cells were identified. Several genes (uhpT, bioA, and lysA) were involved in metabolic processes. The uhpT gene, which encoded a sugar phosphate transporter, was the most frequently isolated gene and was induced by glucose-6-phosphate in vitro. Two of the intracellularly induced genes (pstS and phoA) encode proteins involved in phosphate acquisition and were induced by phosphate limitation in vitro. Additionally, three iron-regulated genes (sufA, sitA, and fhuA) were identified. The sufA promoter was derepressed in iron-limiting media and was also induced by oxidative stress. To determine whether intracellularly induced genes are required for survival or growth in the intracellular environment, we constructed mutations in the S. flexneri uhpT and pstS genes by allelic exchange. The uhpT mutant could not use glucose-6-phosphate as a sole carbon source in vitro but exhibited normal plaque formation on Henle cell monolayers. The pstS mutant had no apparent growth defect in low-phosphate media in vitro but formed smaller plaques on Henle cell monolayers than the parent strain. Both mutants were as effective as the parent strain in inducing apoptosis in a macrophage cell line.
Figures





References
-
- Altuvia, S., D. Weinstein-Fischer, A. Zhang, L. Postow, and G. Storz. 1997. A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90:43-53. - PubMed
-
- Ambudkar, S. V., T. J. Larson, and P. C. Maloney. 1986. Reconstitution of sugar phosphate transport systems of Escherichia coli. J. Biol. Chem. 261:9083-9086. - PubMed
-
- Bennett, R. L., and M. H. Malamy. 1970. Arsenate resistant mutants of Escherichia coli and phosphate transport. Biochem. Biophys. Res. Commun. 40:496-503. - PubMed
-
- Chenais, J., C. Richaud, J. Ronceray, H. Cherest, Y. Surdin-Kerjan, and J. C. Patte. 1981. Construction of hybrid plasmids containing the lysA gene of Escherichia coli: studies of expression in Escherichia coli and Saccharomyces cerevisiae. Mol. Gen. Genet. 182:456-461. - PubMed
-
- Dorman, C. J., and M. E. Porter. 1998. The Shigella virulence gene regulatory cascade: a paradigm of bacterial gene control mechanisms. Mol. Microbiol. 29:677-684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

