Loss of regulatory protein RfaH attenuates virulence of uropathogenic Escherichia coli
- PMID: 12117951
- PMCID: PMC128157
- DOI: 10.1128/IAI.70.8.4406-4413.2002
Loss of regulatory protein RfaH attenuates virulence of uropathogenic Escherichia coli
Abstract
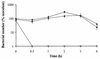
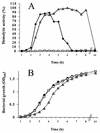
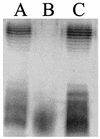
RfaH is a regulatory protein in Escherichia coli and Salmonella enterica serovar Typhimurium. Although it enhances expression of different factors that are proposed to play a role in bacterial virulence, a direct effect of RfaH on virulence has not been investigated so far. We report that inactivation of rfaH dramatically decreases the virulence of uropathogenic E. coli strain 536 in an ascending mouse model of urinary tract infection. The mortality rate caused by the wild-type strain in this assay is 100%, whereas that of its isogenic rfaH mutant does not exceed 18%. In the case of coinfection, the wild-type strain 536 shows higher potential to colonize the urinary tract even when it is outnumbered 100-fold by its rfaH mutant in the inoculum. In contrast to the wild-type strain, serum resistance of strain 536rfaH::cat is fully abolished. Furthermore, we give evidence that, besides a major decrease in the amount of hemin receptor ChuA (G. Nagy, U. Dobrindt, M. Kupfer, L. Emody, H. Karch, and J. Hacker, Infect. Immun. 69:1924-1928, 2001), loss of the RfaH protein results in an altered lipopolysaccharide phenotype as well as decreased expression of K15 capsule and alpha-hemolysin, whereas levels of other pathogenicity factors such as siderophores, flagella, Prf, and S fimbriae appear to be unaltered in strain 536rfaH::cat in comparison to the wild-type strain. trans complementation of the mutant strain with the rfaH gene restores wild-type levels of the affected virulence factors and consequently restitutes virulence in the mouse model of ascending urinary tract infection.
Figures
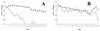
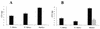
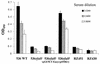
Similar articles
-
The transcriptional antiterminator RfaH represses biofilm formation in Escherichia coli.J Bacteriol. 2006 Feb;188(4):1316-31. doi: 10.1128/JB.188.4.1316-1331.2006. J Bacteriol. 2006. PMID: 16452414 Free PMC article.
-
Expression of hemin receptor molecule ChuA is influenced by RfaH in uropathogenic Escherichia coli strain 536.Infect Immun. 2001 Mar;69(3):1924-8. doi: 10.1128/IAI.69.3.1924-1928.2001. Infect Immun. 2001. PMID: 11179376 Free PMC article.
-
RfaH promotes the ability of the avian pathogenic Escherichia coli O2 strain E058 to cause avian colibacillosis.J Bacteriol. 2013 Jun;195(11):2474-80. doi: 10.1128/JB.02074-12. Epub 2013 Mar 15. J Bacteriol. 2013. PMID: 23504015 Free PMC article.
-
Virulence determinants of uropathogenic Escherichia coli and Proteus mirabilis.Kidney Int Suppl. 1994 Nov;47:S129-36. Kidney Int Suppl. 1994. PMID: 7869662 Review.
-
RfaH and the ops element, components of a novel system controlling bacterial transcription elongation.Mol Microbiol. 1997 Dec;26(5):845-51. doi: 10.1046/j.1365-2958.1997.6432014.x. Mol Microbiol. 1997. PMID: 9426123 Review.
Cited by
-
Ancient Transcription Factors in the News.mBio. 2019 Feb 26;10(1):e01547-18. doi: 10.1128/mBio.01547-18. mBio. 2019. PMID: 30808693 Free PMC article. Review.
-
Phage resistance formation and fitness costs of hypervirulent Klebsiella pneumoniae mediated by K2 capsule-specific phage and the corresponding mechanisms.Front Microbiol. 2023 Jul 19;14:1156292. doi: 10.3389/fmicb.2023.1156292. eCollection 2023. Front Microbiol. 2023. PMID: 37538841 Free PMC article.
-
A new role for Zinc limitation in bacterial pathogenicity: modulation of α-hemolysin from uropathogenic Escherichia coli.Sci Rep. 2018 Apr 25;8(1):6535. doi: 10.1038/s41598-018-24964-1. Sci Rep. 2018. PMID: 29695842 Free PMC article.
-
PafR, a novel transcription regulator, is important for pathogenesis in uropathogenic Escherichia coli.Infect Immun. 2014 Oct;82(10):4241-52. doi: 10.1128/IAI.00086-14. Epub 2014 Jul 28. Infect Immun. 2014. PMID: 25069986 Free PMC article.
-
Molecular basis of commensalism in the urinary tract: low virulence or virulence attenuation?Infect Immun. 2008 Feb;76(2):695-703. doi: 10.1128/IAI.01215-07. Epub 2007 Nov 26. Infect Immun. 2008. PMID: 18039831 Free PMC article.
References
-
- Allison, C., L. Emody, N. Coleman, and C. Hughes. 1994. The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J. Infect. Dis. 169:1155-1158. - PubMed
-
- Bailey, M. J., C. Hughes, and V. Koronakis. 1996. Increased distal gene transcription by the elongation factor RfaH, a specialized homologue of NusG. Mol. Microbiol. 22:729-737. - PubMed
-
- Bailey, M. J., C. Hughes, and V. Koronakis. 1997. RfaH and the ops element, components of a novel system controlling bacterial transcription elongation. Mol. Microbiol. 26:845-851. - PubMed
-
- Bailey, M. J., C. Hughes, and V. Koronakis. 2000. In vitro recruitment of the RfaH regulatory protein into a specialised transcription complex, directed by the nucleic acid ops element. Mol. Gen. Genet. 262:1052-1059. - PubMed
-
- Bailey, M. J., V. Koronakis, T. Schmoll, and C. Hughes. 1992. Escherichia coli HlyT protein, a transcriptional activator of haemolysin synthesis and secretion, is encoded by the rfaH (sfrB) locus required for expression of sex factor and lipopolysaccharide genes. Mol. Microbiol. 6:1003-1012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

