ADP and other metabolites released from Acanthamoeba castellanii lead to human monocytic cell death through apoptosis and stimulate the secretion of proinflammatory cytokines
- PMID: 12117953
- PMCID: PMC128125
- DOI: 10.1128/IAI.70.8.4424-4432.2002
ADP and other metabolites released from Acanthamoeba castellanii lead to human monocytic cell death through apoptosis and stimulate the secretion of proinflammatory cytokines
Erratum in
- Infect Immun 2002 Oct;70(10):5900
Abstract
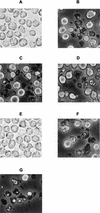
Monocytes/macrophages are thought to be involved in Acanthamoeba infections. The aim of this work was to study whether soluble metabolites (ADP and other compounds) released by Acanthamoeba castellanii trophozoites could induce morphological and biochemical changes in human monocytic cells in vitro. We demonstrate here that ADP constitutively released in the medium by A. castellanii, interacting with specific P2y(2) purinoceptors expressed on the monocytic cell membrane, caused a biphasic rise in [Ca(2+)](i), morphological changes characteristics of cells undergoing apoptosis, caspase-3 activation, and secretion of tumor necrosis factor alpha (TNF-alpha). The same results were found in monocytes exposed to purified ADP. Cell damage and TNF-alpha release induced by amoebic ADP were blocked by the P2y(2) inhibitor suramin. Other metabolites contained in amoebic cell-free supernatants, with molecular masses of, respectively, >30 kDa and between 30 and 10 kDa, also caused morphological modifications and activation of intracellular caspase-3, characteristics of programmed cell death. Nevertheless, mechanisms by which these molecules trigger cell damage appeared to differ from that of ADP. In addition, other amoebic thermolable metabolites with molecular masses of <10 kDa caused the secretion of interleukin-1beta. These findings suggest that pathogenic free-living A. castellanii by release of ADP and other metabolites lead to human monocytic cell death through apoptosis and stimulate the secretion of proinflammatory cytokines.
Figures

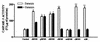

Similar articles
-
By releasing ADP, Acanthamoeba castellanii causes an increase in the cytosolic free calcium concentration and apoptosis in wish cells.Infect Immun. 2001 Jun;69(6):4134-40. doi: 10.1128/IAI.69.6.4134-4140.2001. Infect Immun. 2001. PMID: 11349088 Free PMC article.
-
Acanthamoeba castellanii metabolites increase the intracellular calcium level and cause cytotoxicity in wish cells.Microb Pathog. 1997 Aug;23(2):85-93. doi: 10.1006/mpat.1997.0138. Microb Pathog. 1997. PMID: 9245619
-
Acanthamoeba castellanii Genotype T4 Stimulates the Production of Interleukin-10 as Well as Proinflammatory Cytokines in THP-1 Cells, Human Peripheral Blood Mononuclear Cells, and Human Monocyte-Derived Macrophages.Infect Immun. 2016 Sep 19;84(10):2953-62. doi: 10.1128/IAI.00345-16. Print 2016 Oct. Infect Immun. 2016. PMID: 27481240 Free PMC article.
-
Reverse signaling through transmembrane TNF confers resistance to lipopolysaccharide in human monocytes and macrophages.J Immunol. 2000 Jun 15;164(12):6193-8. doi: 10.4049/jimmunol.164.12.6193. J Immunol. 2000. PMID: 10843670
-
In vitro activity of Acanthamoeba castellanii on human platelets and erythrocytes.Infect Immun. 2009 Feb;77(2):733-8. doi: 10.1128/IAI.00202-08. Epub 2008 Nov 17. Infect Immun. 2009. PMID: 19015256 Free PMC article.
Cited by
-
Acanthamoeba castellanii induces host cell death via a phosphatidylinositol 3-kinase-dependent mechanism.Infect Immun. 2005 May;73(5):2704-8. doi: 10.1128/IAI.73.5.2704-2708.2005. Infect Immun. 2005. PMID: 15845472 Free PMC article.
-
Human Conjunctival Transcriptome in Acanthamoeba Keratitis: An Exploratory Study.Cornea. 2024 Oct 1;43(10):1272-1277. doi: 10.1097/ICO.0000000000003545. Epub 2024 May 21. Cornea. 2024. PMID: 38771726
-
Pathogenic Acanthamoeba castellanii Secretes the Extracellular Aminopeptidase M20/M25/M40 Family Protein to Target Cells for Phagocytosis by Disruption.Molecules. 2017 Dec 18;22(12):2263. doi: 10.3390/molecules22122263. Molecules. 2017. PMID: 29258252 Free PMC article.
-
An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment.Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. Epub 2015 Feb 18. Parasite. 2015. PMID: 25687209 Free PMC article. Review.
-
Extracellular vesicles and vesicle-free secretome of the protozoa Acanthamoeba castellanii under homeostasis and nutritional stress and their damaging potential to host cells.Virulence. 2018 Dec 31;9(1):818-836. doi: 10.1080/21505594.2018.1451184. Virulence. 2018. PMID: 29560793 Free PMC article.
References
-
- Aepfelbacher, M., R. Zumbihl, K. Ruckdeschel, C. A. Jacobi, C. Barz, and J. Heesemann. 1999. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defence. J. Biol. Chem. 380:795-802. - PubMed
-
- Balboa, M. A., J. Balsinde, C. A. Johnson, and A. Dennis. 1999. Regulation of arachidonic acid mobilization in lipopolysaccharide-activated P388D(1) macrophages by adenosine triphosphate. J. Biol. Chem. 274:36764-36768. - PubMed
-
- Berchtold, S., A. L. J. Ogilvie, C. Bogdan, P. Mulh-Zurbes, A. Ogilvie, G. Schuler, and A. Steinkasserer. 1999. Human monocyte derived dendritic cells express functional P2x and P2y receptors as well as ecto-nucleotidase. FEBS Lett. 458:424-428. - PubMed
-
- Byoung-Kuk, N., K. Jae-Chan, and S. Chul-Yong. 2001. Characterization and pathogenetic role of proteinase from Acanthamoeba castellanii. Microb. Pathog. 30:39-48. - PubMed
-
- Clifford, E. E., K. A. Martin, P. Dalal, R. Thomas, and G. Dubyak. 1997. Stage-specific expression of P2y receptors, ecto-apyrase, and ecto-5′-nucleotidase in myeloid leukocytes. Am. J. Physiol. 273:C973-C987. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

