High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion
- PMID: 12117958
- PMCID: PMC128198
- DOI: 10.1128/IAI.70.8.4471-4476.2002
High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion
Erratum in
- Infect Immun 2002 Oct;70(10):5901
Abstract
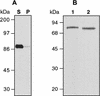
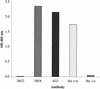
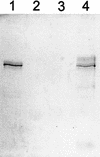
Apical membrane antigen 1 (AMA-1) is a highly promising malaria blood-stage vaccine candidate that has induced protection in rodent and nonhuman primate models of malaria. Authentic conformation of the protein appears to be essential for the induction of parasite-inhibitory antibody responses. Here we have developed a synthetic gene with adapted codon usage to allow expression of Plasmodium falciparum FVO strain AMA-1 (PfAMA-1) in Pichia pastoris. In addition, potential N-glycosylation sites were changed, exploiting the lack of conservation of these sites in Plasmodium, to obtain high-level secretion of a homogeneous product, suitable for scale-up according to current good manufacturing procedures. Purified PfAMA-1 displayed authentic antigenic properties, indicating that the amino acid changes had no deleterious effect on the conformation of the protein. High-titer antibodies, raised in rabbits, reacted strongly with homologous and heterologous P. falciparum by immunofluorescence. In addition, purified immunoglobulin G from immunized animals strongly inhibited invasion of red blood cells by homologous and, to a somewhat lesser extent, heterologous P. falciparum.
Figures
References
-
- Anders, R. F., P. E. Crewther, S. Edwards, M. Margetts, M. L. Matthew, B. Pollock, and D. Pye. 1998. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. - PubMed
-
- Collins, W. E., D. Pye, P. E. Crewther, K. L. Vandenberg, G. G. Galland, A. J. Sulzer, D. J. Kemp, S. J. Edwards, R. L. Coppel, J. S. Sullivan, et al. 1994. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am. J. Trop. Med. Hyg. 51:711-719. - PubMed
-
- Deans, J. A., and W. C. Jean. 1987. Structural studies on a putative protective Plasmodium knowlesi merozoite antigen. Mol. Biochem. Parasitol. 26:155-166. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

