Identification and characterization of a novel heme-associated cell surface protein made by Streptococcus pyogenes
- PMID: 12117961
- PMCID: PMC128137
- DOI: 10.1128/IAI.70.8.4494-4500.2002
Identification and characterization of a novel heme-associated cell surface protein made by Streptococcus pyogenes
Abstract
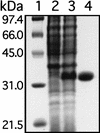
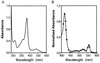
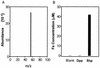
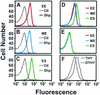
Analysis of the genome sequence of a serotype M1 group A Streptococcus (GAS) strain identified a gene encoding a previously undescribed putative cell surface protein. The gene was cloned from a serotype M1 strain, and the recombinant protein was overexpressed in Escherichia coli and purified to homogeneity. The purified protein was associated with heme in a 1:1 stoichiometry. This streptococcal heme-associated protein, designated Shp, was produced in vitro by GAS, located on the bacterial cell surface, and accessible to specific antibody raised against the purified recombinant protein. Mice inoculated subcutaneously with GAS and humans with invasive infections and pharyngitis caused by GAS seroconverted to Shp, indicating that Shp was produced in vivo. The blood of mice actively immunized with Shp had significantly higher bactericidal activity than the blood of unimmunized mice. The shp gene was cotranscribed with eight contiguous genes, including homologues of an ABC transporter involved in iron uptake in gram-negative bacteria. Our results indicate that Shp is a novel cell surface heme-associated protein.
Figures



Similar articles
-
Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes.Infect Immun. 2003 Oct;71(10):5962-9. doi: 10.1128/IAI.71.10.5962-5969.2003. Infect Immun. 2003. PMID: 14500516 Free PMC article.
-
In vivo efficacy of a chimeric peptide derived from the conserved region of the M protein against group C and G streptococci.Clin Vaccine Immunol. 2012 Dec;19(12):1984-7. doi: 10.1128/CVI.00140-12. Epub 2012 Oct 17. Clin Vaccine Immunol. 2012. PMID: 23081813 Free PMC article.
-
Identification of new candidate vaccine antigens made by Streptococcus pyogenes: purification and characterization of 16 putative extracellular lipoproteins.J Infect Dis. 2004 Jan 1;189(1):79-89. doi: 10.1086/380491. Epub 2003 Dec 22. J Infect Dis. 2004. PMID: 14702157
-
Prospects for a group A streptococcal vaccine.Curr Opin Mol Ther. 2005 Feb;7(1):11-6. Curr Opin Mol Ther. 2005. PMID: 15732524 Review.
-
Strategic development of the conserved region of the M protein and other candidates as vaccines to prevent infection with group A streptococci.Expert Rev Vaccines. 2015;14(11):1459-70. doi: 10.1586/14760584.2015.1081817. Expert Rev Vaccines. 2015. PMID: 26485214 Review.
Cited by
-
Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes.Infect Immun. 2003 Oct;71(10):5962-9. doi: 10.1128/IAI.71.10.5962-5969.2003. Infect Immun. 2003. PMID: 14500516 Free PMC article.
-
Bis-methionyl coordination in the crystal structure of the heme-binding domain of the streptococcal cell surface protein Shp.J Mol Biol. 2007 Nov 23;374(2):374-83. doi: 10.1016/j.jmb.2007.08.058. Epub 2007 Aug 31. J Mol Biol. 2007. PMID: 17920629 Free PMC article.
-
Study of streptococcal hemoprotein receptor (Shr) in iron acquisition and virulence of M1T1 group A streptococcus.Virulence. 2012 Nov 15;3(7):566-75. doi: 10.4161/viru.21933. Epub 2012 Oct 17. Virulence. 2012. PMID: 23076332 Free PMC article.
-
Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence.Infect Immun. 2005 Sep;73(9):5743-53. doi: 10.1128/IAI.73.9.5743-5753.2005. Infect Immun. 2005. PMID: 16113291 Free PMC article.
-
Mechanisms of group A Streptococcus resistance to reactive oxygen species.FEMS Microbiol Rev. 2015 Jul;39(4):488-508. doi: 10.1093/femsre/fuu009. Epub 2015 Feb 10. FEMS Microbiol Rev. 2015. PMID: 25670736 Free PMC article. Review.
References
-
- Drazek, E. S., C. A. Hammack, and M. P. Schmitt. 2000. Corynebacterium diphtheriae genes required for acquisition of iron from haemin and haemoglobin are homologous to ABC haemin transporters. Mol. Microbiol. 36:68-84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

