Identification of Escherichia coli outer membrane protein A receptor on human brain microvascular endothelial cells
- PMID: 12117968
- PMCID: PMC128170
- DOI: 10.1128/IAI.70.8.4556-4563.2002
Identification of Escherichia coli outer membrane protein A receptor on human brain microvascular endothelial cells
Erratum in
- Infect Immun 2002 Nov;70(11):6513
Abstract
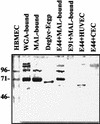
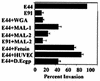
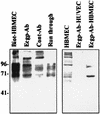
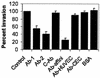
Neonatal Escherichia coli meningitis continues to be a diagnostic and treatment challenge despite the availability of active antibiotics. Our earlier studies have shown that outer membrane protein A (OmpA) is one of the major factors responsible for Escherichia coli traversal across the blood-brain barrier that constitutes a lining of brain microvascular endothelial cells (BMEC). In this study we showed that OmpA binds to a 95-kDa human BMEC (HBMEC) glycoprotein (Ecgp) for E. coli invasion. Ecgp was partially purified by wheat germ agglutinin and Maackia amurensis lectin (MAL) affinity chromatography. The MAL affinity-purified HBMEC proteins bound to OmpA(+) E. coli but not to OmpA(-) E. coli. In addition, the deglycosylated MAL-bound proteins still interact with OmpA(+) E. coli, indicating the role of protein backbone in mediating the OmpA binding to HBMEC. Interestingly, the MAL affinity-bound fraction showed one more protein, a 65-kDa protein that bound to OmpA(+) E. coli in addition to Ecgp. Further, the 65-kDa protein was shown to be a cleavage product of Ecgp. Immunocytochemistry of HBMEC infected with OmpA(+) E. coli by using anti-Ecgp antibody suggests that Ecgp clusters at the E. coli entry site. Anti-Ecgp antibody also reacted to microvascular endothelium on human brain tissue sections, indicating the biological relevance of Ecgp in E. coli meningitis. Partial N-terminal amino acid sequence of Ecgp suggested that it has 87% sequence homology to gp96, an endoplasmic reticulum-resident molecular chaperone that is often expressed on the cell surface. In contrast, the 65-kDa protein, which could be the internal portion of Ecgp, showed 70% sequence homology to an S-fimbria-binding sialoglycoprotein reported earlier. These results suggest that OmpA interacts with Ecgp via the carbohydrate epitope, as well as with the protein portion for invading HBMEC.
Figures


Similar articles
-
Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue.Infect Immun. 2003 Apr;71(4):1680-8. doi: 10.1128/IAI.71.4.1680-1688.2003. Infect Immun. 2003. PMID: 12654781 Free PMC article.
-
Escherichia coli outer membrane protein A adheres to human brain microvascular endothelial cells.Biochem Biophys Res Commun. 2005 May 20;330(4):1199-204. doi: 10.1016/j.bbrc.2005.03.097. Biochem Biophys Res Commun. 2005. PMID: 15823570
-
Identification of minimum carbohydrate moiety in N-glycosylation sites of brain endothelial cell glycoprotein 96 for interaction with Escherichia coli K1 outer membrane protein A.Microbes Infect. 2014 Jul;16(7):540-52. doi: 10.1016/j.micinf.2014.06.002. Epub 2014 Jun 13. Microbes Infect. 2014. PMID: 24932957 Free PMC article.
-
E. coli invasion of brain microvascular endothelial cells as a pathogenetic basis of meningitis.Subcell Biochem. 2000;33:47-59. doi: 10.1007/978-1-4757-4580-1_3. Subcell Biochem. 2000. PMID: 10804851 Review.
-
Current concepts on Escherichia coli K1 translocation of the blood-brain barrier.FEMS Immunol Med Microbiol. 2004 Nov 1;42(3):271-9. doi: 10.1016/j.femsim.2004.09.001. FEMS Immunol Med Microbiol. 2004. PMID: 15477040 Review.
Cited by
-
Virulence and immunomodulatory roles of bacterial outer membrane vesicles.Microbiol Mol Biol Rev. 2010 Mar;74(1):81-94. doi: 10.1128/MMBR.00031-09. Microbiol Mol Biol Rev. 2010. PMID: 20197500 Free PMC article. Review.
-
Entry and intracellular replication of Escherichia coli K1 in macrophages require expression of outer membrane protein A.Infect Immun. 2003 Oct;71(10):5951-61. doi: 10.1128/IAI.71.10.5951-5961.2003. Infect Immun. 2003. PMID: 14500515 Free PMC article.
-
A high-throughput sequencing approach identifies immunotherapeutic targets for bacterial meningitis in neonates.EBioMedicine. 2023 Feb;88:104439. doi: 10.1016/j.ebiom.2023.104439. Epub 2023 Jan 27. EBioMedicine. 2023. PMID: 36709579 Free PMC article.
-
Outer membrane protein A (OmpA) of extraintestinal pathogenic Escherichia coli.BMC Res Notes. 2020 Jan 31;13(1):51. doi: 10.1186/s13104-020-4917-5. BMC Res Notes. 2020. PMID: 32005127 Free PMC article.
-
EHEC Adhesins.Microbiol Spectr. 2014;2(2):EHEC00032013. doi: 10.1128/microbiolspec.EHEC-0003-2013. Microbiol Spectr. 2014. PMID: 25635238 Free PMC article.
References
-
- Bar, R. S., B. L. Dake, and R. G. Rapanheimer. 1985. Sulfated glycosaminoglycans in cultured endothelial cells from capillaries and large vessels of human and bovine origin. Atherosclerosis 56:11-26. - PubMed
-
- Everest, P., J. Li, and G. Douce. 1996. Bordetella pertussis P69/pertactin protein and the P69/pertactin RGD motif in the adherence to and invasion of mammalian cell. Microbiology 142:3261-3268. - PubMed
-
- Feldweg, A. M., and P. K. Srivastava. 1995. Molecular heterogeneity of tumor rejection antigen/heat shock protein GP96. Int. J. Cancer 63:310-314. - PubMed
-
- Gladstone, I. M., R. A. Ehrenkranz, S. C. Edberg, and R. S. Baltimore. 1990. A ten-year review of neonatal sepsis and comparison with previous fifty-year experience. Pediatr. Infect. Dis. J. 9:819-825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

