Fusobacterium necrophorum leukotoxin induces activation and apoptosis of bovine leukocytes
- PMID: 12117974
- PMCID: PMC128195
- DOI: 10.1128/IAI.70.8.4609-4620.2002
Fusobacterium necrophorum leukotoxin induces activation and apoptosis of bovine leukocytes
Abstract
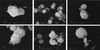
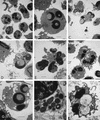
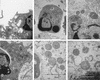
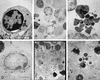
Fusobacterium necrophorum, a gram-negative, rod-shaped, anaerobic bacterium, is a primary or secondary etiological agent in a variety of necrotic, purulent infections in humans and animals. Its major virulence factor is leukotoxin, a high-molecular-weight secreted protein, primarily toxic to ruminant leukocytes. In this study, bovine peripheral blood leukocytes were exposed to various concentrations of immunoaffinity-purified leukotoxin and the cytotoxicity was analyzed by flow cytometry and scanning and transmission electron microscopy. At very low toxin concentrations, polymorphonuclear leukocytes (PMNs) showed activation, as indicated by translocation of primary and secondary granules to the periphery of the cytoplasm. Furthermore, these cells showed changes characteristic of apoptosis, including decreased cell size, organelle condensation, cytoplasmic membrane blebbing (zeiosis), and chromatin condensation and margination, and decrease in cellular DNA content. At moderately high concentrations of leukotoxin, bovine mononuclear cells were also induced to undergo programmed cell death. At very high concentrations, leukotoxin caused necrotic cell death of bovine peripheral leukocytes. The ability of F. necrophorum leukotoxin to modulate the host immune system by its toxicity, including cellular activation of PMNs and apoptosis-mediated killing of phagocytes and immune effector cells, represents a potentially important mechanism of its pathogenesis.
Figures




Similar articles
-
Pasteurella haemolytica leukotoxin induces bovine leukocytes to undergo morphologic changes consistent with apoptosis in vitro.Infect Immun. 1996 Jul;64(7):2687-94. doi: 10.1128/iai.64.7.2687-2694.1996. Infect Immun. 1996. PMID: 8698496 Free PMC article.
-
Characterization of Fusobacterium necrophorum isolated from llama and alpaca.J Vet Diagn Invest. 2013 Jul;25(4):502-7. doi: 10.1177/1040638713491407. Epub 2013 Jun 18. J Vet Diagn Invest. 2013. PMID: 23780933
-
Cloning, sequencing, and expression of the leukotoxin gene from Fusobacterium necrophorum.Infect Immun. 2001 Sep;69(9):5447-55. doi: 10.1128/IAI.69.9.5447-5455.2001. Infect Immun. 2001. PMID: 11500416 Free PMC article.
-
Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy.J Dent Res. 2010 Jun;89(6):561-70. doi: 10.1177/0022034510363682. Epub 2010 Mar 3. J Dent Res. 2010. PMID: 20200418 Free PMC article. Review.
-
Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism and control measures.Vet Res Commun. 1996;20(2):113-40. doi: 10.1007/BF00385634. Vet Res Commun. 1996. PMID: 8711893 Review.
Cited by
-
Enterovirus infection of human islets of Langerhans affects β-cell function resulting in disintegrated islets, decreased glucose stimulated insulin secretion and loss of Golgi structure.BMJ Open Diabetes Res Care. 2016 Aug 10;4(1):e000179. doi: 10.1136/bmjdrc-2015-000179. eCollection 2016. BMJ Open Diabetes Res Care. 2016. PMID: 27547409 Free PMC article.
-
Neutrophil apoptosis and the resolution of infection.Immunol Res. 2009;43(1-3):25-61. doi: 10.1007/s12026-008-8049-6. Immunol Res. 2009. PMID: 19066741 Review.
-
Cell Based Drug Delivery: Micrococcus luteus Loaded Neutrophils as Chlorhexidine Delivery Vehicles in a Mouse Model of Liver Abscesses in Cattle.PLoS One. 2015 May 26;10(5):e0128144. doi: 10.1371/journal.pone.0128144. eCollection 2015. PLoS One. 2015. PMID: 26011247 Free PMC article.
-
Association of pathogenic determinants of Fusobacterium necrophorum with bacteremia, and Lemierre's syndrome.Sci Rep. 2024 Aug 27;14(1):19804. doi: 10.1038/s41598-024-70608-y. Sci Rep. 2024. PMID: 39191804 Free PMC article.
-
Comparative Genomic Analysis of Fusobacterium necrophorum Provides Insights into Conserved Virulence Genes.Microbiol Spectr. 2022 Dec 21;10(6):e0029722. doi: 10.1128/spectrum.00297-22. Epub 2022 Oct 7. Microbiol Spectr. 2022. PMID: 36219094 Free PMC article.
References
-
- Allen, R. C. 1986. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 133:449-493. - PubMed
-
- Allen, R. C., D. C. Dale, and F. B. Taylor, Jr. 2000. Blood phagocyte luminescence: gauging systemic immune activation. Methods Enzymol. 305:591-629. - PubMed
-
- Chen, Y., and A. Zychlinsky. 1994. Apoptosis induced by bacterial pathogens. Microb. Pathog. 17:203-212. - PubMed
-
- Clinkenbeard, K. D., D. A. Mosier, and A. W. Confer. 1989. Effects of Pasteurella haemolytica leukotoxin on isolated bovine neutrophils. Toxicon 27:797-804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

