Hint, Fhit, and GalT: function, structure, evolution, and mechanism of three branches of the histidine triad superfamily of nucleotide hydrolases and transferases
- PMID: 12119013
- PMCID: PMC2571077
- DOI: 10.1021/bi025942q
Hint, Fhit, and GalT: function, structure, evolution, and mechanism of three branches of the histidine triad superfamily of nucleotide hydrolases and transferases
Abstract
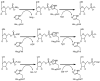
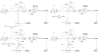
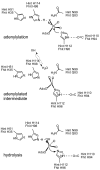
HIT (histidine triad) proteins, named for a motif related to the sequence HphiHphiHphiphi (phi, a hydrophobic amino acid), are a superfamily of nucleotide hydrolases and transferases, which act on the alpha-phosphate of ribonucleotides, and contain a approximately 30 kDa domain that is typically either a homodimer of approximately 15 kDa polypeptides with two active-sites or an internally, imperfectly repeated polypeptide that retains a single HIT active site. On the basis of sequence, substrate specificity, structure, evolution, and mechanism, HIT proteins can be classified into the Hint branch, which consists of adenosine 5'-monophosphoramide hydrolases, the Fhit branch, which consists of diadenosine polyphosphate hydrolases, and the GalT branch, which consists of specific nucleoside monophosphate transferases, including galactose-1-phosphate uridylyltransferase, diadenosine tetraphosphate phosphorylase, and adenylyl sulfate:phosphate adenylytransferase. At least one human representative of each branch is lost in human diseases. Aprataxin, a Hint branch hydrolase, is mutated in ataxia-oculomotor apraxia syndrome. Fhit is lost early in the development of many epithelially derived tumors. GalT is deficient in galactosemia. Additionally, ASW is an avian Hint family member that has evolved to have unusual gene expression properties and the complete loss of its nucleotide binding site. The potential roles of ASW and Hint in avian sexual development are discussed elsewhere. Here we review what is known about biological activities of HIT proteins, the structural and biochemical bases for their functions, and propose a new enzyme mechanism for Hint and Fhit that may account for the differences between HIT hydrolases and transferases.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

