Implications of phase variation of a gene (pgtA) encoding a pilin galactosyl transferase in gonococcal pathogenesis
- PMID: 12119340
- PMCID: PMC2193922
- DOI: 10.1084/jem.20012022
Implications of phase variation of a gene (pgtA) encoding a pilin galactosyl transferase in gonococcal pathogenesis
Abstract
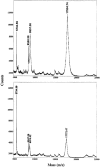
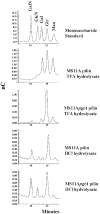
The pilin glycoprotein (PilE) is the main building block of the pilus of Neisseria gonorrhoeae (gonococcus [GC]). GC pilin is known to carry a disaccharide O-glycan, which has an alphaGal attached to the O-linked GlcNAc by a 1-3 glycosidic bond. In this report, we describe the cloning and characterization of the GC gene, pilus glycosyl transferase A (pgtA), which encodes the galactosyl transferase that catalyzes the synthesis of this Gal-GlcNAc bond of pilin glycan. A homopolymeric tract of Gs (poly-G) is present in the pgtA gene of many GC strains, and this pgtA with poly-G can undergo phase variation (Pv). However, in many other GC, pgtA lacks the poly-G and is expressed constitutively without Pv. Furthermore, by screening a large number of clinical isolates, a significant correlation was observed between the presence of poly-G in pgtA and the dissemination of GC infection. Poly-G was found in pgtA in all (24 out of 24) of the isolates from patients with disseminated gonococcal infection (DGI). In contrast, for the vast majority (20 out of 28) of GC isolated from uncomplicated gonorrhea (UG) patients, pgtA lacked the poly-G. These results indicate that Pv of pgtA is likely to be involved in the conversion of UG to DGI.
Figures








References
-
- American Social Health Association. 1998. Sexually Transmitted Diseases in America: How Many Cases and at What Cost? Kaiser Family Foundation, Menlo Park, CA. 27 pp.
-
- Meitzner, T.A., and M.S. Cohen. 1997. Vaccines against gonococcal infection. New Generation Vaccines. M.M. Levin, G.C. Woodrow, J.B. Kaper, and G.S. Cobon, editors. Marcel Dekker, Inc., New York. 817–842.
-
- Anaya, J.M., J. Joseph, E. Scopelitis, and L.R. Espinoza. 1994. Disseminated gonococcal infection and human immunodeficiency virus. Clin. Exp. Rheumatol. 12:688. - PubMed
-
- Jacoby, H.M., and B.J. Mady. 1995. Acute gonococcal sepsis in an HIV-infected woman. Sex. Transm. Dis. 22:380–382. - PubMed
-
- Jonsson, A.B., D. Ilver, P. Falk, J. Pepose, and S. Normark. 1994. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol. Microbiol. 13:403–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

