Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]
- PMID: 12119349
- PMCID: PMC2193931
- DOI: 10.1084/jem.20020642
Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]
Erratum in
- J Exp Med 2002 Aug 19;196(4):559
- J Exp Med 2002 Sep 16;196(6):867
Abstract
It has been recently demonstrated that regulatory CD4(+)CD25(+) CD45RO(+) T cells are present in the peripheral blood of healthy adults and exert regulatory function similar to their rodent counterparts. It remains difficult to understand how the small fraction of these T cells that regulate via direct cell-to-cell contact and not via secretion of immunosuppressive cytokines could mediate strong immune suppression. Here we show that human CD4(+)CD25(+) T cells induce long-lasting anergy and production of interleukin (IL)-10 in CD4(+)CD25(-) T cells. These anergized CD4(+)CD25(-) T cells then suppress proliferation of syngenic CD4(+) T cells via IL-10 but independent of direct cell contact, similar to the so-called type 1 regulatory T (Tr1) cells. This 'catalytic' function of CD4(+)CD25(+) T cells to induce Tr1-like cells helps to explain their central role for the maintenance of immune homeostasis.
Figures






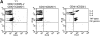
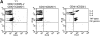
References
-
- Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

