Increased cell proliferation, but not reduced cell death, induces lymphocytosis in bovine leukemia virus-infected sheep
- PMID: 12119390
- PMCID: PMC126622
- DOI: 10.1073/pnas.142100999
Increased cell proliferation, but not reduced cell death, induces lymphocytosis in bovine leukemia virus-infected sheep
Abstract
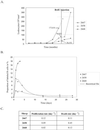
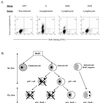
Lymphocyte homeostasis is the result of a critical balance between cell proliferation and death. Disruption of this subtle equilibrium can lead to the onset of leukemia, an increase in the number of lymphocytes being potentially due to both of these parameters. The relative importance of cell proliferation vs. apoptosis during pathogenesis induced by the primate T cell lymphotropic viruses and bovine leukemia virus (BLV) has been difficult to assess because of conflicting data from a range of in vitro and ex vivo experimental systems. Here, we aim to resolve this issue by measuring the rates of cell proliferation and death in the BLV-ovine system, an animal model of human T lymphotropic virus (HTLV-1). We use a method based on the i.v. injection of 5-bromodeoxyuridine into BLV-infected sheep. We show that B lymphocytes in BLV(+) asymptomatic sheep proliferate significantly faster than in uninfected controls (average proliferation rate: 0.020 per day vs. 0.011 per day). In contrast, the rates of cell death were not significantly different between aleukemic BLV-infected and control sheep (average death rate 0.089 per day vs. 0.094 per day, respectively). We conclude that the increase in the number of B cells during BLV-induced lymphocytosis results from higher proliferation rates but is not due to a significant decrease in apoptosis, in contrast to data from in vitro (ex vivo) experiments. The imbalance created by the net increase in proliferation in the absence of compensating cell death reveals a complex mechanism of feedback regulation controlling homeostasis in the blood compartment.
Figures


References
-
- Gallo R C. Nat Med. 1995;1:753–759. - PubMed
-
- Yoshida M. Gann. 1983;74:777–789. - PubMed
-
- Ferrer J F, Marshak R R, Abt D A, Kenyon S J. Ann Rech Vet. 1978;9:851–857. - PubMed
-
- Burny A, Cleuter Y, Kettmann R, Mammerickx M, Marbaix G, Portetelle D, Van den Broecke A, Willems L, Thomas R. Adv Vet Sci Comp Med. 1988;32:149–170. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

