Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells
- PMID: 12119403
- PMCID: PMC125028
- DOI: 10.1073/pnas.152205299
Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells
Abstract
A high degree of aneuploidy characterizes the majority of human tumors. Aneuploid status can arise through mitotic or cleavage failure coupled with failure of tetraploid G(1) checkpoint control, or through deregulation of centrosome number, thus altering the number of mitotic spindle poles. p53 and the RB pocket proteins are important to the control of G(1) progression, and p53 has previously been suggested as important to the control of centrosome duplication. We demonstrate here that neither suppression of p53 nor of the RB pocket protein family directly generates altered centrosome numbers in any of several mammalian primary cell lines. Instead, amplification of centrosome number occurs in two steps. The first step is failure to arrest at a G(1) tetraploidy checkpoint after failure to segregate the genome in mitosis, and the second step is clustering of centrosomes at a single spindle pole in subsequent tetraploid or aneuploid mitosis. The trigger for these events is mitotic or cleavage failure that is independent of p53 or RB status. Finally, we find that mouse embryo fibroblasts spontaneously enter tetraploid G(1), explaining the previous demonstration of centrosome amplification by p53 abrogation alone in these cells.
Figures

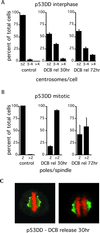


References
-
- Cahill D P, Lengauer C, Yu J, Riggins G J, Willson J K, Markowitz S D, Kinzler K W, Vogelstein B. Nature (London) 1998;392:300–303. - PubMed
-
- Sandberg A A. Cancer Res. 1977;37:2950–2956. - PubMed
-
- Rabinovitch P S, Reid B J, Haggitt R C, Norwood T H, Rubin C E. Lab Invest. 1989;60:65–71. - PubMed
-
- Giaretti W. Lab Invest. 1994;71:904–910. - PubMed
-
- Schutte B, Reynders M M, Wiggers T, Arends J W, Volovics L, Bosman F T, Blijham G H. Cancer Res. 1987;47:5494–5496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

