Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer
- PMID: 12119406
- PMCID: PMC126640
- DOI: 10.1073/pnas.152259999
Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer
Abstract
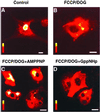
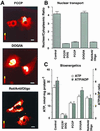
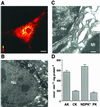
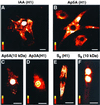
Exchange of information between the nucleus and cytosol depends on the metabolic state of the cell, yet the energy-supply pathways to the nuclear compartment are unknown. Here, the energetics of nucleocytoplasmic communication was determined by imaging import of a constitutive nuclear protein histone H1. Translocation of H1 through nuclear pores in cardiac cells relied on ATP supplied by mitochondrial oxidative phosphorylation, but not by glycolysis. Although mitochondria clustered around the nucleus, reducing the distance for energy transfer, simple nucleotide diffusion was insufficient to meet the energetic demands of nuclear transport. Rather, the integrated phosphotransfer network was required for delivery of high-energy phosphoryls from mitochondria to the nucleus. In neonatal cardiomyocytes with low creatine kinase activity, inhibition of adenylate kinase-catalyzed phosphotransfer abolished nuclear import. With deficient adenylate kinase, nucleoside diphosphate kinase, which secures phosphoryl exchange between ATP and GTP, was unable to sustain nuclear import. Up-regulation of creatine kinase phosphotransfer, to mimic metabolic conditions of adult cardiac cells, rescued H1 import, suggesting a developmental plasticity of the cellular energetic system. Thus, mitochondrial oxidative phosphorylation coupled with phosphotransfer relays provides an efficient energetic unit in support of nuclear transport.
Figures
References
-
- Mattaj I W, Englmeier L. Annu Rev Biochem. 1998;67:265–306. - PubMed
-
- Gorlich D, Kutay U. Annu Rev Cell Dev Biol. 1999;15:607–660. - PubMed
-
- Perez-Terzic C, Pyle J, Jaconi M, Stehno-Bittel L, Clapham D E. Science. 1996;273:1875–1877. - PubMed
-
- Perez-Terzic C, Gacy A M, Bortolon R, Dzeja P P, Puceat M, Jaconi M, Prendergast F G, Terzic A. Circ Res. 1999;84:1292–12301. - PubMed
-
- Talcott B, Moore M S. Trends Cell Biol. 1999;9:312–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

