Crystal structure of monomeric human beta-2-microglobulin reveals clues to its amyloidogenic properties
- PMID: 12119416
- PMCID: PMC125010
- DOI: 10.1073/pnas.152337399
Crystal structure of monomeric human beta-2-microglobulin reveals clues to its amyloidogenic properties
Abstract
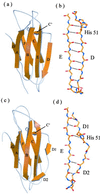
Dissociation of human beta-2-microglobulin (beta(2)m) from the heavy chain of the class I HLA complex is a critical first step in the formation of amyloid fibrils from this protein. As a consequence of renal failure, the concentration of circulating monomeric beta(2)m increases, ultimately leading to deposition of the protein into amyloid fibrils and development of the disorder, dialysis-related amyloidosis. Here we present the crystal structure of a monomeric form of human beta(2)m determined at 1.8-A resolution that reveals remarkable structural changes relative to the HLA-bound protein. These involve the restructuring of a beta bulge that separates two short beta strands to form a new six-residue beta strand at one edge of this beta sandwich protein. These structural changes remove key features proposed to have evolved to protect beta sheet proteins from aggregation [Richardson, J. & Richardson, D. (2002) Proc. Natl. Acad. Sci. USA 99, 2754-2759] and replaces them with an aggregation-competent surface. In combination with solution studies using (1)H NMR, we show that the crystal structure presented here represents a rare species in solution that could provide important clues about the mechanism of amyloid formation from the normally highly soluble native protein.
Figures

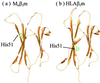
References
-
- Saper M A, Bjorkman P J, Wiley D C. J Mol Biol. 1991;219:277–319. - PubMed
-
- Floege J, Ehlerding G. Nephron. 1996;72:9–26. - PubMed
-
- Linke R P, Bommer J, Ritz E, Waldherr R, Eulitz M. Biochem Biophys Res Commun. 1986;136:665–671. - PubMed
-
- Bellotti V, Stoppini M, Mangione P, Sunde M, Robinson C, Asti L, Brancaccio D, Ferri G. Eur J Biochem. 1998;258:61–67. - PubMed
-
- Lai Z H, Colon W, Kelly J W. Biochemistry. 1996;35:6470–6482. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

