Association of calnexin with mutant peripheral myelin protein-22 ex vivo: a basis for "gain-of-function" ER diseases
- PMID: 12119418
- PMCID: PMC125041
- DOI: 10.1073/pnas.152621799
Association of calnexin with mutant peripheral myelin protein-22 ex vivo: a basis for "gain-of-function" ER diseases
Abstract
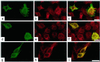
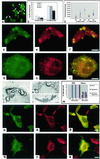
Schwann cell-derived peripheral myelin protein-22 (PMP-22) when mutated or overexpressed causes heritable neuropathies with a previously unexplained "gain-of-function" endoplasmic reticulum (ER) retention phenotype. In wild-type sciatic nerves, PMP-22 associates in a specific, transient (t(1/2 ) approximately equal to 11 min), and oligosaccharide processing-dependent manner with the lectin chaperone calnexin (CNX), but not calreticulin nor BiP. In Trembler-J (Tr-J) sciatic nerves, prolonged association of mutant PMP-22 with CNX is found (t(1/2) > 60 min). In 293A cells overexpressing PMP-22(Tr-J), CNX and PMP-22 colocalize in large intracellular structures identified at the electron microscopy level as myelin-like figures with CNX localization in the structures dependent on PMP-22 glucosylation. Similar intracellular myelin-like figures were also present in Schwann cells of sciatic nerves from homozygous Trembler-J mice with no detectable activation of the stress response pathway as deduced from BiP and CHOP expression. Sequestration of CNX in intracellular myelin-like figures may be relevant to the autosomal dominant Charcot-Marie-Tooth-related neuropathies.
Figures


References
-
- Naef R, Suter U. Microsc Res Tech. 1998;41:359–371. - PubMed
-
- Lupski J R, de Oca-Luna R M, Slaugenhaupt S, Pentao L, Guzzetta V, Trask B J, Saucedo-Cardenas O, Barker D F, Killian J M, Garcia C A, et al. Cell. 1991;66:219–232. - PubMed
-
- Chance P F, Alderson M K, Leppig K A, Lensch M W, Matsunami N, Smith B, Swanson P D, Odelberg S J, Disteche C M, Bird T D. Cell. 1993;72:143–151. - PubMed
-
- Warner L E, Garcia C A, Lupski J R. Annu Rev Med. 1999;50:263–275. - PubMed
-
- Suter U, Welcher A A, Ozcelik T, Snipes G J, Kosaras B, Francke U, Billings-Gagliardi S, Sidman R L, Shooter E M. Nature (London) 1992;356:241–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

