Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action
- PMID: 12121906
- PMCID: PMC127361
- DOI: 10.1128/AAC.46.8.2365-2372.2002
Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action
Abstract
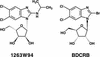
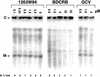
Benzimidazole nucleosides have been shown to be potent inhibitors of human cytomegalovirus (HCMV) replication in vitro. As part of the exploration of structure-activity relationships within this series, we synthesized the 2-isopropylamino derivative (3322W93) of 1H-beta-D-ribofuranoside-2-bromo-5,6-dichlorobenzimidazole (BDCRB) and the biologically unnatural L-sugars corresponding to both compounds. One of the L derivatives, 1H-beta-L-ribofuranoside-2-isopropylamino-5,6-dichlorobenzimidazole (1263W94), showed significant antiviral potency in vitro against both laboratory HCMV strains and clinical HCMV isolates, including those resistant to ganciclovir (GCV), foscarnet, and BDCRB. 1263W94 inhibited viral replication in a dose-dependent manner, with a mean 50% inhibitory concentration (IC(50)) of 0.12 +/- 0.01 microM compared to a mean IC(50) for GCV of 0.53 +/- 0.04 microM, as measured by a multicycle DNA hybridization assay. In a single replication cycle, 1263W94 treatment reduced viral DNA synthesis, as well as overall virus yield. HCMV mutants resistant to 1263W94 were isolated, establishing that the target of 1263W94 was a viral gene product. The resistance mutation was mapped to the UL97 open reading frame. The pUL97 protein kinase was strongly inhibited by 1263W94, with 50% inhibition occurring at 3 nM. Although HCMV DNA synthesis was inhibited by 1263W94, the inhibition was not mediated by the inhibition of viral DNA polymerase. The parent benzimidazole D-riboside BDCRB inhibits viral DNA maturation and processing, whereas 1263W94 does not. The mechanism of the antiviral effect of L-riboside 1263W94 is thus distinct from those of GCV and of BDCRB. In summary, 1263W94 inhibits viral replication by a novel mechanism that is not yet completely understood.
Figures


References
-
- Baldanti, F., E. Silini, A. Sarasini, C. Talarico, S. Stanat, K. Biron, M. Furione, F. Bono, G. Palu, and G. Gerna. 1995. A three-nucleotide deletion in the UL97 open reading frame is responsible for the ganciclovir resistance of a human cytomegalovirus clinical isolate. J. Virol. 69:796-800. - PMC - PubMed
-
- Baldanti, F., M. Underwood, S. Stanat, K. Biron, S. Chou, A. Sarasini, E. Silini, and G. Gerna. 1996. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J. Virol. 70:1390-1395. - PMC - PubMed
-
- Blanchard, J. 1981. Evaluation of the relative efficacy of various techniques for deproteinizing plasma samples prior to high-performance liquid chromatographic analysis. J. Chromatogr. 226:455-460. - PubMed
-
- Chamberlain, S., S. Daluge, and G. Koszalka. June 2000. Antiviral benzimidazole nucleoside analogues and a method for their preparation. U.S. patent 6077832.
-
- Chan, J., S. Chamberlain, K. Biron, M. Davis, R. Harvey, D. Selleseth, R. Dornsife, E. Dark, L. Frick, L. Townsend, J. Drach, and G. Koszalka. 2000. Synthesis and evaluation of a series of 2′-deoxy analogues of the antiviral agent 5,6-dichloro-2-isopropylamino-1-(β-l-ribofuranosyl)-1H-benzimidazole (1263W94). Nucleosides Nucleotides Nucleic Acids 19:101-123. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

