Identification of small-molecule inhibitors of nucleoside triphosphate hydrolase in Toxoplasma gondii
- PMID: 12121910
- PMCID: PMC127337
- DOI: 10.1128/AAC.46.8.2393-2399.2002
Identification of small-molecule inhibitors of nucleoside triphosphate hydrolase in Toxoplasma gondii
Abstract
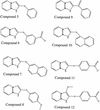
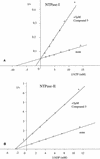
Approximately 150,000 small-molecule compounds were tested by a robotic screening assay for their ability to inhibit nucleoside triphosphate hydrolase (NTPase), a novel enzyme of the tachyzoite form of Toxoplasma gondii. Five unrelated species of compounds were found to inhibit the activities of both NTPase isoforms (NTPase isoform I [NTPase-I] and NTPase-II). The 50% inhibitory concentrations (IC(50)s) ranged from 0.1 to 20 microM, and in general, the IC(50)s were similar for both NTPase isoforms. However, the activity of NTPase-I was 20 times more sensitive than the activity of NTPase-II to one of the inhibitors: 9-hydroxy-10-(pentachlorophenoxy)stearic acid. The five compounds identified also prevented tachyzoite replication in vitro, with IC(50)s ranging from approximately 7 to > or =50 microM. The most effective of these initial compounds, 2-phenylthio-indole, was used to identify six additional, structurally related compounds, which were tested for their inhibitory effects on enzyme activities and tachyzoite replication. Surprisingly, these compounds were competitive inhibitors of NTPase-I but noncompetitive inhibitors of NTPase-II. Modifications to the indole and phenol rings resulted in alterations of activity, thus providing insight into the structural features that are important for inhibition of T. gondii NTPases.
Figures

References
-
- Asai, T., D. K. Howe, K. Nakajima, T. Nozaki, T. Takeuchi, and L. D. Sibley. 1998. Neospora caninum: tachyzoites express a potent type-I nucleoside triphosphate hydrolase, but lack nucleoside diphosphate hydrolase activity. Exp. Parasitol. 90:277-285. - PubMed
-
- Asai, T., S. Miura, L. D. Sibley, H. Okabayashi, and T. Takeuchi. 1995. Biochemical and molecular characterization of nucleoside triphosphate hydrolase isozymes from the parasitic protozoan Toxoplasma gondii. J. Biol. Chem. 270:11391-11397. - PubMed
-
- Asai, T., W. J. O'Sullivan, and M. Tatibana. 1983. A potent nucleoside triphosphate hydrolase from the parasitic protozoan Toxoplasma gondii. J. Biol. Chem. 258:6816-6822. - PubMed
-
- Beaman, M. H., B. J. Luft, and J. S. Remington. 1992. Assessment of therapy for toxoplasmic encephalitis. Ann. Intern. Med. 117:163-164. - PubMed
-
- Bermudes, D., K. R. Peck, M. A. Afifi, C. J. M. Beckers, and K. A. Joiner. 1994. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J. Biol. Chem. 269:29252-29260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

