Inhibition of cyclin-dependent kinase 1 by purines and pyrrolo[2,3-d]pyrimidines does not correlate with antiviral activity
- PMID: 12121920
- PMCID: PMC127371
- DOI: 10.1128/AAC.46.8.2470-2476.2002
Inhibition of cyclin-dependent kinase 1 by purines and pyrrolo[2,3-d]pyrimidines does not correlate with antiviral activity
Abstract
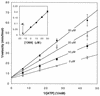
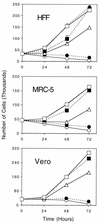
We have previously shown that a series of nonnucleoside pyrrolo[2,3-d]pyrimidines selectively inhibit the replication of herpes simplex virus type 1 (HSV-1) and human cytomegalovirus (HCMV). These compounds act at the immediate-early or early stage of HCMV replication and have antiviral properties somewhat similar to those of roscovitine and olomoucine, specific inhibitors of cyclin-dependent kinases (cdks). In the present study we examine the hypothesis that pyrrolo[2,3-d]pyrimidines exert their antiviral effects by inhibition of cellular cdks. Much higher concentrations of a panel of pyrrolo[2,3-d]pyrimidine nucleoside analogs with antiviral activity were required to inhibit recombinant cdk1/cyclin B compared to the submicromolar concentrations required to inhibit HCMV and HSV-1 replication. 4,6-Diamino-5-cyano-7-(2-phenylethyl)pyrrolo[2,3-d]pyrimidine (compound 1369) was the best inhibitor of cdk1 and cyclin B, with a 50% inhibitory concentration (IC(50); 14 microM) similar to that of roscovitine; it was competitive with respect to ATP (K(i) = 14 microM). The potency of compound 1369 against cdk1 and cyclin B was similar to its cytotoxicity (IC(50)s, 32 to 100 microM) but not its antiviral efficacy (IC(50)s, 0.02 to 0.3 microM). Thus, our results indicated the null hypothesis. In contrast, roscovitine was only weakly active against HSV-1 (IC(50), 38 microM) and HCMV (IC(50), 40 microM). These values were similar to those derived by cytotoxicity and cell growth inhibition assays, thereby suggesting that roscovitine is not a selective antiviral. Therefore, we propose that inhibition of cdk1 and cyclin B is not responsible for selective antiviral activity and that pyrrolo[2,3-d]pyrimidines constitute novel pharmacophores which compete with ATP to inhibit cdk1 and cyclin B.
Figures


References
-
- Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. Disappearance of cyclins A and B and the increase in activity of G2/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the α22/US1.5 and UL13 viral genes. J. Virol. 74:8-15. - PMC - PubMed
-
- Alford, C. A., and W. J. Britt. 1993. Cytomegalovirus, p. 227-255. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, New York, N.Y.
-
- Breshnahan, W. E., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. - PubMed
-
- Breshnahan, W. E., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular cdk2 activity blocks human cytomegalovirus replication. Virology 231:239-247. - PubMed
-
- Breshnahan, W. E., E. A. Thompson, and T. Albrecht. 1997. Human cytomegalovirus infection results in altered cdk2 subcellular localization. J. Gen. Virol. 78:1993-1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

