Automatic postural responses are delayed by pyridoxine-induced somatosensory loss
- PMID: 12122040
- PMCID: PMC6757909
- DOI: 10.1523/JNEUROSCI.22-14-05803.2002
Automatic postural responses are delayed by pyridoxine-induced somatosensory loss
Abstract
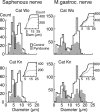
Pyridoxine given in large doses is thought to destroy selectively the large-diameter peripheral sensory nerve fibers, leaving motor fibers intact. This study examined the effects of pyridoxine-induced somatosensory loss on automatic postural responses to sudden displacements of the support surface in the standing cat. Two cats were trained to stand on four force plates mounted on a movable platform. They were given pyridoxine (350 mg/kg, i.p.) on 2 successive days (0 and 1). Electromyographic (EMG) activity was recorded from selected hindlimb muscles during linear ramp-and-hold platform displacements in each of 12 directions at 15 cm/sec. In control trials onset latencies of evoked activity in hindlimb flexor and extensor muscles ranged from 40 to 65 msec after the onset of platform acceleration. After injection the EMG latencies increased over days, becoming two to three times longer than controls by day 7. Excursions of the body center of mass (CoM) in the direction opposite to that of platform translation were significantly greater at day 7 compared with controls, and the time at which the CoM subsequently reversed direction was delayed. Both animals were ataxic from day 2 onward. Histological analysis of cutaneous and muscle nerves in the hindlimb revealed a significant loss of fibers in the group I range. Our results suggest that large afferent fibers are critical for the timing of automatic postural responses to ensure coordinated control of the body CoM and balance after unexpected disturbances of the support surface.
Figures


References
-
- Allum JHJ, Bloem BR, Carpenter MG, Hulliger M, Hadders-Algra M. Proprioceptive control of posture: a review of new concepts. Gait Posture. 1998;8:214–242. - PubMed
-
- Boyd IA, Davey MR. Composition of peripheral nerves. Livingstone; Edinburgh: 1968.
-
- Diener HC, Dichgans J, Guschlbauer B, Mau H. The significance of proprioception on postural stabilization as assessed by ischemia. Brain Res. 1984;296:103–109. - PubMed
-
- Diener HC, Horak FB, Nashner LM. Influence of stimulus parameters on human postural responses. J Neurophysiol. 1988;59:1888–1905. - PubMed
-
- Do MC, Bussel B, Breniere Y. Influence of plantar cutaneous afferents on early compensatory reactions to forward fall. Exp Brain Res. 1990;79:319–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
