Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster
- PMID: 12122057
- PMCID: PMC6757919
- DOI: 10.1523/JNEUROSCI.22-14-05946.2002
Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster
Abstract
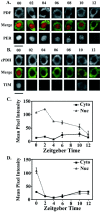
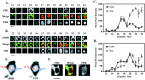
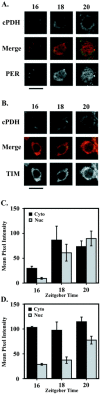
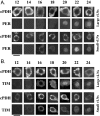
Antisera against the circadian clock proteins Period (PER) and Timeless (TIM) were used to construct a detailed time course of PER and TIM expression and subcellular localization in a subset of the ventrolateral neurons (vLNs) in the Drosophila accessory medulla (AMe). These neurons, which express pigment-dispersing factor, play a central role in the control of behavioral rhythms. The data revealed several unexpected features of the circadian clock in Drosophila. First, TIM but not PER was restricted to the cytoplasm of vLNs throughout most of the early night. Second, the timing of TIM and PER nuclear accumulation was substantially different. Third, the two subsets of vLNs, the large and small vLNs, had a similar timing of PER nuclear accumulation but differed by 3-4 hr in the phase of TIM nuclear accumulation. These aspects of PER and TIM expression were not predicted by the current mechanistic model of the circadian clock in Drosophila and are inconsistent with the hypothesis that PER and TIM function as obligate heterodimers. The differing profiles of TIM and PER nuclear accumulation suggest that PER and TIM have distinct functions in the nuclei of vLNs.
Figures


References
-
- Allada R, White NE, So WV, Hall JC, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. - PubMed
-
- Curtin KD, Huang ZJ, Rosbash M. Temporally regulated nuclear entry of the Drosophila Period protein contributes to the circadian clock. Neuron. 1995;14:365–372. - PubMed
-
- Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TDL, Weitz CJ, Takahashi JS, Kay SA. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. - PubMed
-
- Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP. The ultrastructure of nerve endings containing the pigment-dispersing hormone (PDH) in crustacean sinus glands: identification by an antiserum against synthetic PDH. Cell Tissue Res. 1987;250:377–387.
-
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
