The type 1 interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury
- PMID: 12122068
- PMCID: PMC6757935
- DOI: 10.1523/JNEUROSCI.22-14-06071.2002
The type 1 interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury
Abstract
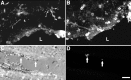
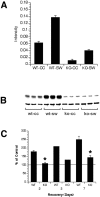
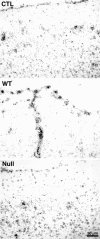
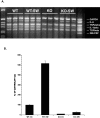
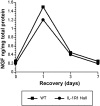
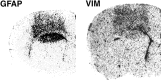
Interleukin-1 (IL-1) is induced immediately after insults to the brain, and elevated levels of IL-1 have been strongly implicated in the neurodegeneration that accompanies stroke, Alzheimer's disease, and multiple sclerosis. In animal models, antagonizing IL-1 has been shown to reduce cell death; however, the basis for this protection has not been elucidated. Here we analyzed the response to penetrating brain injury in mice lacking the type 1 IL-1 receptor (IL-1R1) to determine which cellular and molecular mediators of tissue damage require IL-1 signaling. At the cellular level, fewer amoeboid microglia/macrophages appeared adjacent to the injured brain tissue in IL-1R1 null mice, and those microglia present at early postinjury intervals retained their resting morphology. Astrogliosis also was mildly abrogated. At the molecular level, cyclooxygenase-2 (Cox-2) and IL-6 expression were depressed and delayed. Interestingly, basal levels of Cox-2, IL-1, and IL-6 were significantly lower in the IL-1R1 null mice. In addition, stimulation of vascular cell adhesion molecule-1 mRNA was depressed in the IL-1R1 null mice, and correspondingly, there was reduced diapedesis of peripheral macrophages in the IL-1R1 null brain after injury. This observation correlated with a reduced number of Cox-2+ amoeboid phagocytes adjacent to the injury. In contrast, several molecular aspects of the injury response were normal, including expression of tumor necrosis factor-alpha and the production of nerve growth factor. Because antagonizing IL-1 protects neural cells in experimental models of stroke and multiple sclerosis, our data suggest that cell preservation is achieved by abrogating microglial/macrophage activation and the subsequent self-propagating cycle of inflammation.
Figures



References
-
- Acarin L, Vela JM, González B, Castellano B. Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding. J Histochem Cytochem. 1994;42:1033–1041. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of FGF-2, to increase motor neuron survival. Exp Neurol. 2002;173:46–62. - PubMed
-
- Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1β and tumor necrosis factor-α. J Immunol. 1992;149:2358–2366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
