Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor
- PMID: 12122113
- PMCID: PMC151066
- DOI: 10.1172/JCI15745
Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor
Abstract
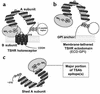
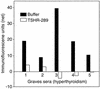
Graves disease is directly caused by thyroid-stimulating autoantibodies (TSAb's) that activate the thyrotropin receptor (TSHR). We observed upon flow cytometry using intact cells that a mouse mAb (3BD10) recognized the TSHR ectodomain with a glycosidylphosphatidylinositol (ECD-GPI) anchor approximately tenfold better than the same ectodomain on the wild-type TSHR, despite the far higher level of expression of the latter. The 3BD10 epitope contains the N-terminal cysteine cluster critical for TSAb action. Consequently, we hypothesized and confirmed that TSAb (but not thyrotropin-blocking autoantibodies [TBAb's]) also poorly recognize the wild-type TSHR relative to the ECD-GPI. Despite poor recognition by TSAb of the holoreceptor, soluble TSHR A subunits (known to be shed from surface TSHR) fully neutralized autoantibody-binding activity. These data indicate that the epitope(s) for TSAb's, but not for TBAb's, are partially sterically hindered on the holoreceptor by the plasma membrane, the serpentine region of the TSHR, or by TSHR dimerization. However, the TSAb epitope on the soluble A subunit is freely accessible. This observation, as well as other evidence, supports the concept that A subunit shedding either initiates or amplifies the autoimmune response to the TSHR, thereby causing Graves disease in genetically susceptible individuals.
Figures
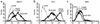


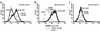
Comment in
-
The TSH receptor reveals itself.J Clin Invest. 2002 Jul;110(2):161-4. doi: 10.1172/JCI16234. J Clin Invest. 2002. PMID: 12122107 Free PMC article. Review. No abstract available.
References
-
- Zakarija M. Immunochemical characterization of the thyroid-stimulating antibody (TSAb) of Graves’ disease: evidence for restricted heterogeneity. J Clin Lab Immunol. 1983;10:77–85. - PubMed
-
- Knight J, Laing P, Knight A, Adams D, Ling N. Thyroid stimulating autoantibodies usually contain only lambda-light chains: evidence for the “forbidden clone” theory. J Clin Endocrinol Metab. 1986;62:342–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

