Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome
- PMID: 12122117
- PMCID: PMC151050
- DOI: 10.1172/JCI15058
Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome
Erratum in
- J Clin Invest 2002 Oct;110(8):1213
Abstract
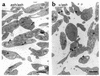
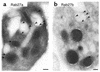
Griscelli syndrome (GS) patients and the corresponding mouse model ashen exhibit defects mainly in two types of lysosome-related organelles, melanosomes in melanocytes and lytic granules in CTLs. This disease is caused by loss-of-function mutations in RAB27A, which encodes 1 of the 60 known Rab GTPases, critical regulators of vesicular transport. Here we present evidence that Rab27a function can be compensated by a closely related protein, Rab27b. Rab27b is expressed in platelets and other tissues but not in melanocytes or CTLs. Morphological and functional tests in platelets derived from ashen mice are all within normal limits. Both Rab27a and Rab27b are found associated with the limiting membrane of platelet-dense granules and to a lesser degree with alpha-granules. Ubiquitous transgenic expression of Rab27a or Rab27b rescues ashen coat color, and melanocytes derived from transgenic mice exhibit widespread peripheral distribution of melanosomes instead of the perinuclear clumping observed in ashen melanocytes. Finally, transient expression in ashen melanocytes of Rab27a or Rab27b, but not other Rab's, restores peripheral distribution of melanosomes. Our data suggest that Rab27b is functionally redundant with Rab27a and that the pathogenesis of GS is determined by the relative expression of Rab27a and Rab27b in specialized cell types.
Figures

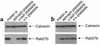



References
-
- Pereira-Leal JB, Seabra MC. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol. 2000;301:1077–1087. - PubMed
-
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. - PubMed
-
- Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol. 2001;13:500–511. - PubMed
-
- Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. - PubMed
-
- Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

