Spatial characteristics of sarcoplasmic reticulum Ca2+ release events triggered by L-type Ca2+ current and Na+ current in guinea-pig cardiac myocytes
- PMID: 12122139
- PMCID: PMC2290414
- DOI: 10.1113/jphysiol.2001.013382
Spatial characteristics of sarcoplasmic reticulum Ca2+ release events triggered by L-type Ca2+ current and Na+ current in guinea-pig cardiac myocytes
Abstract
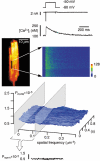
Ca2+ signals in cardiac muscle cells are composed of spatially limited elementary events termed Ca2+ sparks. Several studies have also indicated that Ca2+ signals smaller than Ca2+ sparks can be elicited. These signals have been termed Ca2+ quarks and were proposed to result from the opening of a single Ca2+ release channel of the sarcoplasmic reticulum. We used laser-scanning confocal microscopy to examine the subcellular properties of Na+ current (I(Na))- and L-type Ca2+ current (I(Ca,L))-induced Ca2+ transients in voltage-clamped ventricular myocytes isolated from guinea-pigs. Both currents, I(Na) and I(Ca,L), evoked substantial, global Ca2+ transients. To examine the spatiotemporal properties of such Ca2+ signals, we performed power spectral analysis of these Ca2+ transients and found that both lacked spatial frequency components characteristic for Ca2+ sparks. The application of 10 microM verapamil to partially block L-type Ca2+ current reduced the corresponding Ca2+ transients down to individual Ca2+ sparks. In contrast, I(Na)-induced Ca2+ responses were still spatially homogeneous and lacked Ca2+ sparks even for small current amplitudes. By using high resistance patch pipettes (> 4 MOmega) to exaggerate the loss of voltage control during I(Na), Ca2+ sparks appeared superimposed on a homogeneous Ca2+ release component and were exclusively triggered during the flow of I(Na). In the presence of 10 microM ryanodine both I(Ca,L) and I(Na) elicited small, residual Ca2+ transients that were spatially homogeneous but displayed distinctively different temporal profiles. We conclude that I(Na) is indeed able to cause Ca2+ release in guinea-pig ventricular myocytes. In contrast to I(Ca,L)-induced Ca2+ transients, which are built up from the recruitment of individual Ca2+ sparks, the I(Na)-evoked cellular responses were always homogeneous, indicating that their underlying elementary Ca2+ release event is distinct from the Ca2+ spark. Thus, I(Na)-induced Ca2+ transients are composed of smaller Ca2+ signals, most likely Ca2+ quarks.
Figures


Similar articles
-
Fundamental calcium release events revealed by two-photon excitation photolysis of caged calcium in Guinea-pig cardiac myocytes.J Physiol. 1998 May 1;508 ( Pt 3)(Pt 3):801-9. doi: 10.1111/j.1469-7793.1998.801bp.x. J Physiol. 1998. PMID: 9518734 Free PMC article.
-
Submicroscopic calcium signals as fundamental events of excitation--contraction coupling in guinea-pig cardiac myocytes.J Physiol. 1996 Apr 1;492 ( Pt 1)(Pt 1):31-8. doi: 10.1113/jphysiol.1996.sp021286. J Physiol. 1996. PMID: 8730580 Free PMC article.
-
Local Ca(2+) transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder.J Physiol. 2001 Jul 15;534(Pt. 2):313-26. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x. J Physiol. 2001. PMID: 11454953 Free PMC article.
-
Imaging microdomain Ca2+ in muscle cells.Circ Res. 2004 Apr 30;94(8):1011-22. doi: 10.1161/01.RES.0000125883.68447.A1. Circ Res. 2004. PMID: 15117829 Review.
-
Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone.Acta Physiol Scand. 1998 Dec;164(4):577-87. doi: 10.1046/j.1365-201X.1998.00462.x. Acta Physiol Scand. 1998. PMID: 9887980 Review.
Cited by
-
Mini-dystrophin expression down-regulates IP3-mediated calcium release events in resting dystrophin-deficient muscle cells.J Gen Physiol. 2006 Aug;128(2):219-30. doi: 10.1085/jgp.200609559. Epub 2006 Jul 17. J Gen Physiol. 2006. PMID: 16847098 Free PMC article.
-
Mutation of the calmodulin binding motif IQ of the L-type Ca(v)1.2 Ca2+ channel to EQ induces dilated cardiomyopathy and death.J Biol Chem. 2012 Jun 29;287(27):22616-25. doi: 10.1074/jbc.M112.357921. Epub 2012 May 15. J Biol Chem. 2012. PMID: 22589547 Free PMC article.
-
Cardiac-type EC-coupling in dysgenic myotubes restored with Ca2+ channel subunit isoforms alpha1C and alpha1D does not correlate with current density.Biophys J. 2003 Jun;84(6):3816-28. doi: 10.1016/S0006-3495(03)75109-1. Biophys J. 2003. PMID: 12770887 Free PMC article.
-
Glial glutamate transporter and glutamine synthetase regulate GABAergic synaptic strength in the spinal dorsal horn.J Neurochem. 2012 May;121(4):526-36. doi: 10.1111/j.1471-4159.2012.07694.x. Epub 2012 Mar 21. J Neurochem. 2012. PMID: 22339645 Free PMC article.
-
Ca sparks do not explain all ryanodine receptor-mediated SR Ca leak in mouse ventricular myocytes.Biophys J. 2010 May 19;98(10):2111-20. doi: 10.1016/j.bpj.2010.01.042. Biophys J. 2010. PMID: 20483318 Free PMC article.
References
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001.
-
- Bers DM, Fill M. Muscle contraction — Coordinated feet and the dance of ryanodine receptors. Science. 1998;281:790–791. - PubMed
-
- Bustamante JO. Block of sodium currents by the calcium antagonist D600 in human heart cell segments. Pflügers Archiv. 1985;403:225–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

