Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles
- PMID: 12122145
- PMCID: PMC2316149
- DOI: 10.1113/jphysiol.2002.018630
Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles
Abstract
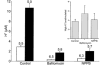
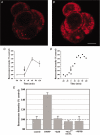
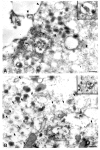
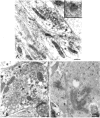
Trapping of weak bases was utilized to evaluate stimulus-induced changes in the internal pH of the secretory vesicles of chromaffin cells and enteric neurons. The internal acidity of chromaffin vesicles was increased by the nicotinic agonist 1,1-dimethyl-4-phenyl-piperazinium iodide (DMPP; in vivo and in vitro) and by high K+ (in vitro); and in enteric nerve terminals by exposure to veratridine or a plasmalemmal [Ca2+]o receptor agonist (Gd3+). Stimulation-induced acidification of chromaffin vesicles was [Ca2+]o-dependent and blocked by agents that inhibit the vacuolar proton pump (vH+-ATPase) or flux through Cl- channels. Stimulation also increased the average volume of chromaffin vesicles and the proportion that displayed a clear halo around their dense cores (called active vesicles). Stimulation-induced increases in internal acidity and size were greatest in active vesicles. Stimulation of chromaffin cells in the presence of a plasma membrane marker revealed that membrane was internalized in endosomes but not in chromaffin vesicles. The stable expression of botulinum toxin E to prevent exocytosis did not affect the stimulation-induced acidification of the secretory vesicles of mouse neuroblastoma Neuro2A cells. Stimulation-induced acidification thus occurs independently of exocytosis. The quantal size of secreted catecholamines, measured by amperometry in cultured chromaffin cells, was found to be increased either by prior exposure to L-DOPA or stimulation by high K+, and decreased by inhibition of vH+-ATPase or flux through Cl- channels. These observations are consistent with the hypothesis that the content of releasable small molecules in secretory vesicles is increased when the driving force for their uptake is enhanced, either by increasing the transmembrane concentration or pH gradients.
Figures





References
-
- Aguado F, Gombau L, Majo G, Marsal J, Blanco J, Blasi J. Regulated secretion is impaired in AtT-20 endocrine cells stably transfected with botulinum neurotoxin type A light chain. Journal of Biological Chemistry. 1997;272:26005–26008. - PubMed
-
- Anderson RGW, Pathak RK. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985;40:635–643. - PubMed
-
- Aspinwall CA, Brooks SA, Kennedy RT, Lakey JR. Effects of intravesicular H+ and extracellular H+ and Zn2+ on insulin secretion in pancreatic beta cells. Journal of Biological Chemistry. 1997;272:31308–31314. - PubMed
-
- Bae H-R, Verkman AS. Protein kinase A regulates chloride conductance in endocytic vesicles from proximal tubule. Nature. 1990;348:637–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

