Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man
- PMID: 12122159
- PMCID: PMC2290411
- DOI: 10.1113/jphysiol.2001.015644
Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man
Abstract
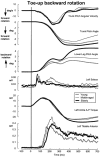
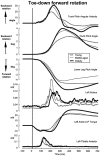
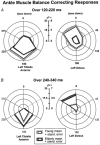
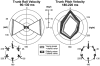
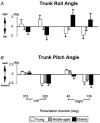
We investigated the effects of ageing on balance corrections induced by sudden stance perturbations in different directions. Effects were examined in biomechanical and electromyographic (EMG) recordings from a total of 36 healthy subjects divided equally into three age groups (20-34, 35-55 and 60-75 years old). Perturbations consisted of six combinations of support-surface roll (laterally) and pitch (forward-backward) each with 7.5 deg amplitude (2 pure pitch, and 4 roll and pitch) delivered randomly. To reduce stimulus predictability further and to investigate scaling effects, perturbations were at either 30 or 60 deg s(-1). In the legs, trunk and arms we observed age-related changes in balance corrections. The changes that appeared in the lower leg responses included smaller stretch reflexes in soleus and larger reflexes in tibialis anterior of the elderly compared with the young. For all perturbation directions, onsets of balance correcting responses in these ankle muscles were delayed by 20-30 ms and initially had smaller amplitudes (between 120-220 ms) in the elderly. This reduced early activity was compensated by increased lower leg activity after 240 ms. These EMG changes were paralleled by comparable differences in ankle torque responses, which were initially (after 160 ms) smaller in the elderly, but subsequently greater (after 280 ms). Findings in the middle-aged group were generally intermediate between the young and the elderly groups. Comparable results were obtained for the two different stimulus velocities. Stimulus-induced trunk roll, but not trunk pitch, changed dramatically with increasing age. Young subjects responded with early large roll movements of the trunk in the opposite direction to platform roll. A similarly directed but reduced amplitude of trunk roll was observed in the middle-aged. The elderly had very little initial roll modulation and also had smaller stretch reflexes in paraspinals. Balance-correcting responses (over 120-220 ms) in gluteus medius and paraspinals were equally well tuned to roll in the elderly, as in the young, but were reduced in amplitude. Onset latencies were delayed with age in gluteus medius muscles. Following the onset of trunk and hip balance corrections, trunk roll was in the same direction as support-surface motion for all age groups and resulted in overall trunk roll towards the fall side in the elderly, but not in the young. Protective arm movements also changed with age. Initial arm roll movements were largest in the young, smaller in the middle aged, and smallest in the elderly. Initial arm roll movements were in the same direction as initial trunk motion in the young and middle aged. Thus initial roll arm movements in the elderly were directed oppositely to those in the young. Initial pitch motion of the arms was similar across age groups. Subsequent arm movements were related to the amplitude of deltoid muscle responses which commenced at 100 ms in the young and 20-30 ms later in the elderly. These deltoid muscle responses preceded additional arm roll motion which left the arms directed 'downhill' (in the direction of the fall) in the elderly, but 'uphill' (to counterbalance motion of the pelvis) in the young. We conclude that increased trunk roll stiffness is a key biomechanical change with age. This interferes with early compensatory trunk movements and leads to trunk displacements in the direction of the impending fall. The reversal of protective arm movements in the elderly may reflect an adaptive strategy to cushion the fall. The uniform delay and amplitude reduction of balance-correcting responses across many segments (legs, hips and arms) suggests a neurally based alteration in processing times and response modulation with age. Interestingly, the elderly compensated for these 'early abnormalities' with enlarged later responses in the legs, but no similar adaptation was noted in the arms and trunk. These changes with age provide an insight into possible mechanisms underlying falls in the elderly.
Figures






References
-
- Accornero N, Capozza M, Rinalduzzi S, Manfredi GW. Clinical multisegmental posturography: age-related changes in stance control. Electroencephalography and Clinical Neurophysiology. 1997;105:213–219. - PubMed
-
- Adkin AL, Frank JS, Peysar GW, Carpenter MG. Fear of falling modifies anticipatory postural control. Experimental Brain Research. 2002;143:160–170. - PubMed
-
- Alexander NB, Shepard N, Gu MJ, Schultz A. Postural control in young and elderly adults when stance is perturbed: Kinematics. Journal of Gerontology. 1992;47:M79. - PubMed
-
- Allum JHJ, Honegger F, Schicks H. Vestibular and proprioceptive modulation of postural synergies in normal subjects. Journal of Vestibular Research. 1993;3:59–85. - PubMed
-
- Allum JHJ, Mauritz K-H. Compensation for intrinsic muscle stiffness by short-latency reflexes in human triceps surae. Journal of Neurophysiology. 1984;52:797–818. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

