alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease
- PMID: 12122208
- PMCID: PMC125054
- DOI: 10.1073/pnas.152339799
alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease
Abstract
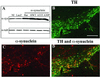
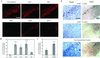
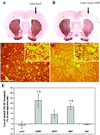
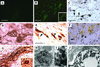
Parkinson's disease (PD) is characterized by the progressive loss of substantia nigra dopaminergic neurons and the presence of cytoplasmic inclusions named Lewy bodies. Two missense mutations of the alpha-synuclein (alpha-syn; A30P and A53T) have been described in several families with an autosomal dominant form of PD. alpha-Syn also constitutes one of the main components of Lewy bodies in sporadic cases of PD. To develop an animal model of PD, lentiviral vectors expressing different human or rat forms of alpha-syn were injected into the substantia nigra of rats. In contrast to transgenic mice models, a selective loss of nigral dopaminergic neurons associated with a dopaminergic denervation of the striatum was observed in animals expressing either wild-type or mutant forms of human alpha-syn. This neuronal degeneration correlates with the appearance of abundant alpha-syn-positive inclusions and extensive neuritic pathology detected with both alpha-syn and silver staining. Lentiviral-mediated expression of wild-type or mutated forms of human alpha-syn recapitulates the essential neuropathological features of PD. Rat alpha-syn similarly leads to protein aggregation but without cell loss, suggesting that inclusions are not the primary cause of cell degeneration in PD. Viral-mediated genetic models may contribute to elucidate the mechanism of alpha-syn-induced cell death and allow the screening of candidate therapeutic molecules.
Figures
References
-
- Polymeropoulos M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. (1997) Science 276, 2045-2047. - PubMed
-
- Kruger R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L. & Riess, O. (1998) Nat. Genet. 18, 106-108. - PubMed
-
- Spillantini M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R. & Goedert, M. (1997) Nature (London) 388, 839-840. - PubMed
-
- Iwai A., Masliah, E., Yoshimoto, M., Ge, N., Flanagan, L., de Silva, H. A., Kittel, A. & Saitoh, T. (1995) Neuron 14, 467-475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

