Impact of reference-based pricing for histamine-2 receptor antagonists and restricted access for proton pump inhibitors in British Columbia
- PMID: 12126319
- PMCID: PMC116151
Impact of reference-based pricing for histamine-2 receptor antagonists and restricted access for proton pump inhibitors in British Columbia
Abstract
Background: Two programs to reduce expenditures for common gastrointestinal drugs were introduced simultaneously by British Columbia (BC) Pharmacare in 1995. Reference-based pricing restricted reimbursement for all histamine-2 receptor antagonists (H2RAs) to the cost of the least expensive H2RA available, generic cimetidine. Special authority restricted reimbursement for proton pump inhibitors (PPIs) to patients who met certain eligibility criteria. We evaluated the effect of reference-based pricing for H2RAs and special authority for PPIs on dispensing and reimbursement for senior citizen beneficiaries of BC Pharmacare.
Methods: Itemized monthly claims data for upper gastrointestinal drugs were obtained from BC Pharmacare for all beneficiaries 65 years of age or older. Periods before and after implementation of reference-based pricing and special authority were compared with respect to defined daily doses dispensed per 100,000 beneficiaries, BC Pharmacare reimbursement per 100,000 beneficiaries, BC Pharmacare reimbursement per defined daily dose and beneficiary contributions per defined daily dose. We used regression models to project forward trends in expenditures observed before implementation of the new policies and hence to estimate accrued cost savings.
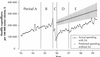
Results: Before reference-based pricing and special authority, the numbers of defined daily doses that were dispensed and total BC Pharmacare reimbursements for H2RAs appeared to be declining gradually, whereas those for PPIs were rising. With reference-based pricing, the monthly defined daily dose of cimetidine dispensed increased more than 4-fold, to 116,257 per 100,000 beneficiaries, while those of other restricted H2RAs decreased by more than half, to 50,927 per 100,000 beneficiaries. Special authority immediately reduced the dispensed volumes of PPIs by one-fourth, but growth in volume then appeared to resume at its previous rate. The estimated annualized cost savings achieved by reference-based pricing and special authority were $1.8 million to $3.2 million for H2RAs (depending on the estimation method used) and $5.5 million for PPIs. However, beneficiary contributions for H2RAs increased from negligible amounts to approximately 16% of total drug expenditures.
Interpretation: Reference-based pricing and special authority appear to have been successful in altering prescribing habits and reducing provincial expenditures for upper gastrointestinal drugs, but they have increased the financial burden on senior citizen beneficiaries.
Comment in
-
Restricted access for PPIs not a panacea.CMAJ. 2002 Nov 12;167(10):1102; author reply 1102-4. CMAJ. 2002. PMID: 12427695 Free PMC article. No abstract available.
References
-
- Grootendorst P, Holbrook A. Evaluating the impact of reference-based pricing. CMAJ 1999;161:273-4. Available: www.cma.ca/cmaj/vol-161/issue-3/0273.htm - PMC - PubMed
-
- Dickson M, Redwood H. Pharmaceutical reference prices: How do they work in practice? Pharmacoeconomics 1998;14:471-9. - PubMed
-
- Narine L, Senathirajah M, Smith T. Evaluating reference-based pricing: initial findings and prospects. CMAJ 1999;161:286-8. Available: www.cma.ca/cmaj/vol-161/issue-3/0286.htm - PMC - PubMed
-
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment. Oslo: World Health Organization; 2000.
-
- Table 051-0001: Estimates of population, by age group and sex, Canada, provinces and territories, annual (persons unless otherwise noted). In: Statistics Canada's socio-economic database (CANSIM II). Ottawa: Statistics Canada; 2002. Data available through: cansim2.statcan.ca/cii/cii_1_e.htm (accessed 2002 Apr 17).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous