Avian reoviruses cause apoptosis in cultured cells: viral uncoating, but not viral gene expression, is required for apoptosis induction
- PMID: 12133997
- PMCID: PMC155131
- DOI: 10.1128/jvi.76.16.7932-7941.2002
Avian reoviruses cause apoptosis in cultured cells: viral uncoating, but not viral gene expression, is required for apoptosis induction
Abstract
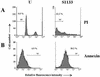
The cytopathic effect evidenced by cells infected with avian reovirus S1133 suggests that this virus may induce apoptosis in primary cultures of chicken embryo fibroblasts. In this report we present evidence that avian reovirus infection of cultured cells causes activation of the intracellular apoptotic program and that this activation takes place during an early stage of the viral life cycle. The ability of avian reoviruses to induce apoptosis is not restricted to a particular virus strain or to a specific cell type, since different avian reovirus isolates were able to induce apoptosis in several avian and mammalian cell lines. Apoptosis was also provoked in ribavirin-treated avian reovirus-infected cells and in cells infected with UV-irradiated reovirions, indicating that viral mRNA synthesis and subsequent steps in viral replication are not needed for apoptosis induction in avian reovirus-infected cells and that the number of inoculated virus particles, not their infectivity, is the critical factor for apoptosis induction by avian reovirus. Our finding that apoptosis is no longer induced when intracellular viral uncoating is blocked indicates that intraendosomal virion disassembly is required for apoptosis induction and that attachment and uptake of parental reovirions are not sufficient to cause apoptosis. Taken together, our results suggest that apoptosis is triggered from within the infected cell by viral products generated after intraendosomal uncoating of parental reovirions.
Figures





Similar articles
-
Early steps in avian reovirus morphogenesis.Curr Top Microbiol Immunol. 2006;309:67-85. doi: 10.1007/3-540-30773-7_3. Curr Top Microbiol Immunol. 2006. PMID: 16909897 Review.
-
The avian reovirus genome segment S1 is a functionally tricistronic gene that expresses one structural and two nonstructural proteins in infected cells.Virology. 2001 Nov 25;290(2):181-91. doi: 10.1006/viro.2001.1159. Virology. 2001. PMID: 11883183
-
Optimal conditions for the growth, purification and storage of the avian reovirus S1133.J Virol Methods. 2000 Mar;85(1-2):43-54. doi: 10.1016/s0166-0934(99)00155-x. J Virol Methods. 2000. PMID: 10716337
-
Interferon induction by avian reovirus.Virology. 2016 Jan;487:104-11. doi: 10.1016/j.virol.2015.10.009. Epub 2015 Oct 23. Virology. 2016. PMID: 26517397
-
Reovirus receptors, cell entry, and proapoptotic signaling.Adv Exp Med Biol. 2013;790:42-71. doi: 10.1007/978-1-4614-7651-1_3. Adv Exp Med Biol. 2013. PMID: 23884585 Free PMC article. Review.
Cited by
-
Inhibition of reovirus by mycophenolic acid is associated with the M1 genome segment.J Virol. 2004 Jun;78(12):6171-9. doi: 10.1128/JVI.78.12.6171-6179.2004. J Virol. 2004. PMID: 15163710 Free PMC article.
-
Trial watch: Oncolytic viruses for cancer therapy.Oncoimmunology. 2013 Jun 1;2(6):e24612. doi: 10.4161/onci.24612. Epub 2013 Apr 16. Oncoimmunology. 2013. PMID: 23894720 Free PMC article.
-
Bluetongue virus outer capsid proteins are sufficient to trigger apoptosis in mammalian cells.J Virol. 2004 Mar;78(6):2875-83. doi: 10.1128/jvi.78.6.2875-2883.2004. J Virol. 2004. PMID: 14990706 Free PMC article.
-
Reconstruction of Avian Reovirus History and Dispersal Patterns: A Phylodynamic Study.Viruses. 2024 May 16;16(5):796. doi: 10.3390/v16050796. Viruses. 2024. PMID: 38793677 Free PMC article.
-
Avian reovirus-triggered apoptosis enhances both virus spread and the processing of the viral nonstructural muNS protein.Virology. 2014 Aug;462-463:49-59. doi: 10.1016/j.virol.2014.04.039. Epub 2014 Jun 17. Virology. 2014. PMID: 25092461 Free PMC article.
References
-
- Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

