Alpha interferon inhibits hepatitis C virus replication in primary human hepatocytes infected in vitro
- PMID: 12134024
- PMCID: PMC155162
- DOI: 10.1128/jvi.76.16.8189-8199.2002
Alpha interferon inhibits hepatitis C virus replication in primary human hepatocytes infected in vitro
Abstract
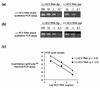
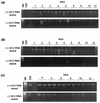
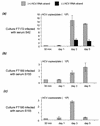
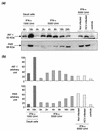
Chronic hepatitis C is a common cause of liver disease, the complications of which include cirrhosis and hepatocellular carcinoma. Treatment of chronic hepatitis C is based on the use of alpha interferon (IFN-alpha). Recently, indirect evidence based on mathematical modeling of hepatitis C virus (HCV) dynamics during human IFN-alpha therapy suggested that the major initial effect of IFN-alpha is to block HCV virion production or release. Here, we used primary cultures of healthy, uninfected human hepatocytes to show that: (i) healthy human hepatocytes can be infected in vitro and support HCV genome replication, (ii) hepatocyte treatment with IFN-alpha results in expression of IFN-alpha-induced genes, and (iii) IFN-alpha inhibits HCV replication in infected human hepatocytes. These results show that IFN-alpha acts primarily through its nonspecific antiviral effects and suggest that primary cultures of human hepatocytes may provide a good model to study intrinsic HCV resistance to IFN-alpha.
Figures


Similar articles
-
ME3738 enhances the effect of interferon and inhibits hepatitis C virus replication both in vitro and in vivo.J Hepatol. 2011 Jul;55(1):11-8. doi: 10.1016/j.jhep.2010.10.017. Epub 2010 Nov 29. J Hepatol. 2011. PMID: 21145867
-
Hydroxyurea as an inhibitor of hepatitis C virus RNA replication.Arch Virol. 2010 Apr;155(4):601-5. doi: 10.1007/s00705-010-0624-1. Epub 2010 Mar 5. Arch Virol. 2010. PMID: 20204428
-
Persistence of HCV in quiescent hepatic cells under conditions of an interferon-induced antiviral response.Gastroenterology. 2012 Aug;143(2):429-38.e8. doi: 10.1053/j.gastro.2012.04.018. Epub 2012 Apr 19. Gastroenterology. 2012. PMID: 22522091
-
Replication of the hepatitis C virus in cell culture.Antiviral Res. 2003 Oct;60(2):91-102. doi: 10.1016/j.antiviral.2003.08.016. Antiviral Res. 2003. PMID: 14638404 Review.
-
Life style-related diseases of the digestive system: cell culture system for the screening of anti-hepatitis C virus (HCV) reagents: suppression of HCV replication by statins and synergistic action with interferon.J Pharmacol Sci. 2007 Oct;105(2):145-50. doi: 10.1254/jphs.fm0070050. Epub 2007 Oct 6. J Pharmacol Sci. 2007. PMID: 17928739 Review.
Cited by
-
L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus.Proc Natl Acad Sci U S A. 2004 Sep 28;101(39):14067-72. doi: 10.1073/pnas.0405695101. Epub 2004 Sep 15. Proc Natl Acad Sci U S A. 2004. PMID: 15371595 Free PMC article.
-
CD56+ T cells inhibit hepatitis C virus replication in human hepatocytes.Hepatology. 2009 Mar;49(3):753-62. doi: 10.1002/hep.22715. Hepatology. 2009. PMID: 19085952 Free PMC article.
-
Primary hepatocytes as targets for hepatitis C virus replication.J Viral Hepat. 2008 Dec;15(12):849-54. doi: 10.1111/j.1365-2893.2008.01051.x. J Viral Hepat. 2008. PMID: 19087224 Free PMC article. Review.
-
Intracellular immunity: finding the enemy within--how cells recognize and respond to intracellular pathogens.J Leukoc Biol. 2014 Aug;96(2):233-44. doi: 10.1189/jlb.4RI0214-090R. Epub 2014 Jun 4. J Leukoc Biol. 2014. PMID: 24899588 Free PMC article.
-
MAIT cells are activated during human viral infections.Nat Commun. 2016 Jun 23;7:11653. doi: 10.1038/ncomms11653. Nat Commun. 2016. PMID: 27337592 Free PMC article.
References
-
- Agnello, V., G. Abel, G. B. Knight, and E. Muchmore. 1998. Detection of widespread hepatocyte infection in chronic hepatitis C. Hepatology 28:573-584. - PubMed
-
- Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. - PubMed
-
- Chieux, V., D. Hober, J. Harvey, G. Lion, D. Lucidarme, G. Forzy, M. Duhamel, J. Cousin, H. Ducoulombier, and P. Wattre. 1998. The MxA protein levels in whole blood lysates of patients with various viral infections. J. Virol. Methods 70:183-191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

