Inhibition of chemokine expression by adenovirus early region three (E3) genes
- PMID: 12134029
- PMCID: PMC155150
- DOI: 10.1128/jvi.76.16.8236-8243.2002
Inhibition of chemokine expression by adenovirus early region three (E3) genes
Abstract
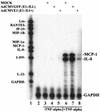
Adenoviruses (Ad) have a variety of immunoregulatory genes, many of which are clustered in a 3.5-kb segment of DNA known as early region 3 (E3). Ad E3 codes for proteins that downregulate surface expression of class I major histocompatibility antigens and also inhibit tumor necrosis factor alpha (TNF-alpha)- and Fas-induced cytolysis. We were interested in determining whether chemokine production or activity might also be inhibited by Ad E3 and we have studied this function in a human astrocytoma cell line, U373. Astrocytes constitute a part of the blood-brain barrier, and chemokines (IP-10, IL-8, MCP-1-4, and MIPs) expressed by them may contribute to leukocyte infiltration within the brain during inflammation. When U373 cells are activated by the proinflammatory molecule TNF-alpha, the increase in chemokine MCP-1, IL-8, and IP-10 transcripts is blocked by a recombinant Ad expressing the E3 genes under cytomegalovirus promoter control. Comparable Ads expressing green fluorescent protein in place of E3 have no effect on these chemokines. Ads also have been extensively studied as gene therapy vectors and most have a deletion of the E3 region to permit the insertion of larger fragments of foreign DNA. Our results suggest that construction of Ad vectors to include E3 expression cassettes will improve the efficacy and safety of such viral-based gene therapy protocols.
Figures




References
-
- Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. - PubMed
-
- Amin, R., R. Wilmott, Y. Schwarz, B. Trapnell, and J. Stark. 1995. Replication-deficient adenovirus induces expression of interleukin-8 by airway epithelial cells in vitro. Hum. Gene Ther. 6:145-153. - PubMed
-
- Bennett, E. M., J. R. Bennink, J. W. Yewdell, and F. M. Brodsky. 1999. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J. Immunol. 162:5049-5052. - PubMed
-
- Bodaghi, B., T. R. Jones, D. Zipeto, C. Vita, L. Sun, L. Laurent, F. Arenzana-Seisdedos, J. L. Virelizier, and S. Michelson. 1998. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J. Exp. Med. 188:855-866. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

