The block in assembly of modified vaccinia virus Ankara in HeLa cells reveals new insights into vaccinia virus morphogenesis
- PMID: 12134037
- PMCID: PMC155139
- DOI: 10.1128/jvi.76.16.8318-8334.2002
The block in assembly of modified vaccinia virus Ankara in HeLa cells reveals new insights into vaccinia virus morphogenesis
Abstract
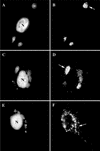
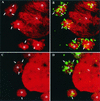
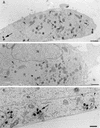
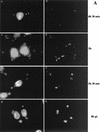
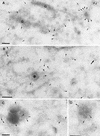
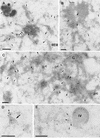
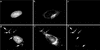
It has previously been shown that upon infection of HeLa cells with modified vaccinia virus Ankara (MVA), assembly is blocked at a late stage of infection and immature virions (IVs) accumulate (G. Sutter and B. Moss, Proc. Natl. Acad. Sci. USA 89:10847-10851, 1992). In the present study the morphogenesis of MVA in HeLa cells was studied in more detail and compared to that under two conditions that permit the production of infectious particles: infection of HeLa cells with the WR strain of vaccinia virus (VV) and infection of BHK cells with MVA. Using several quantitative and qualitative assays, we show that early in infection, MVA in HeLa cells behaves in a manner identical to that under the permissive conditions. By immunofluorescence microscopy (IF) at late times of infection, the labelings for an abundant membrane protein of the intracellular mature virus, p16/A14L, and the viral DNA colocalize under permissive conditions, whereas in HeLa cells infected with MVA these two structures do not colocalize to the same extent. In both permissive and nonpermissive infection, p16-labeled IVs first appear at 5 h postinfection. In HeLa cells infected with MVA, IVs accumulated predominantly outside the DNA regions, whereas under permissive conditions they were associated with the viral DNA. At 4 h 30 min, the earliest time at which p16 is detected, the p16 labeling was found predominantly in a small number of distinct puncta by IF, which were distinct from the sites of DNA in both permissive and nonpermissive infection. By electron microscopy, no crescents or IVs were found at this time, and the p16-labeled structures were found to consist of membrane-rich vesicles that were in continuity with the cellular endoplasmic reticulum. Over the next 30 min of infection, a large number of p16-labeled crescents and IVs appeared abruptly under both permissive and nonpermissive conditions. Under permissive conditions, these IVs were in close association with the sites of DNA, and a significant amount of these IVs engulfed the viral DNA. In contrast, under nonpermissive conditions, the IVs and DNA were mostly in separate locations and relatively few IVs acquired DNA. Our data show that in HeLa cells MVA forms normal DNA replication sites and normal viral precursor membranes but the transport between these two structures is inhibited.
Figures



Similar articles
-
Differences in virus-induced cell morphology and in virus maturation between MVA and other strains (WR, Ankara, and NYCBH) of vaccinia virus in infected human cells.J Virol. 2003 Oct;77(19):10606-22. doi: 10.1128/jvi.77.19.10606-10622.2003. J Virol. 2003. PMID: 12970445 Free PMC article.
-
Plasma membrane budding as an alternative release mechanism of the extracellular enveloped form of vaccinia virus from HeLa cells.J Virol. 2003 Sep;77(18):9931-42. doi: 10.1128/jvi.77.18.9931-9942.2003. J Virol. 2003. PMID: 12941903 Free PMC article.
-
Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line.Virology. 1997 Nov 24;238(2):198-211. doi: 10.1006/viro.1997.8845. Virology. 1997. PMID: 9400593
-
Poxvirus membrane biogenesis.Virology. 2015 May;479-480:619-26. doi: 10.1016/j.virol.2015.02.003. Epub 2015 Feb 26. Virology. 2015. PMID: 25728299 Free PMC article. Review.
-
Assembly of vaccinia virus revisited: de novo membrane synthesis or acquisition from the host?Trends Microbiol. 2002 Jan;10(1):15-24. doi: 10.1016/s0966-842x(01)02256-9. Trends Microbiol. 2002. PMID: 11755081 Review.
Cited by
-
Differences in virus-induced cell morphology and in virus maturation between MVA and other strains (WR, Ankara, and NYCBH) of vaccinia virus in infected human cells.J Virol. 2003 Oct;77(19):10606-22. doi: 10.1128/jvi.77.19.10606-10622.2003. J Virol. 2003. PMID: 12970445 Free PMC article.
-
Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells.J Virol. 2004 Jun;78(11):5820-34. doi: 10.1128/JVI.78.11.5820-5834.2004. J Virol. 2004. PMID: 15140980 Free PMC article.
-
Plasma membrane budding as an alternative release mechanism of the extracellular enveloped form of vaccinia virus from HeLa cells.J Virol. 2003 Sep;77(18):9931-42. doi: 10.1128/jvi.77.18.9931-9942.2003. J Virol. 2003. PMID: 12941903 Free PMC article.
-
Involvement of the cellular phosphatase DUSP1 in vaccinia virus infection.PLoS Pathog. 2013;9(11):e1003719. doi: 10.1371/journal.ppat.1003719. Epub 2013 Nov 14. PLoS Pathog. 2013. PMID: 24244156 Free PMC article.
-
Vaccinia Virus Infection Inhibits Skin Dendritic Cell Migration to the Draining Lymph Node.J Immunol. 2021 Feb 15;206(4):776-784. doi: 10.4049/jimmunol.2000928. Epub 2021 Jan 8. J Immunol. 2021. PMID: 33419767 Free PMC article.
References
-
- Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. - PubMed
-
- Beaud, G., and R. Beaud. 1997. Preferential virosomal location of underphosphorylated H5R protein synthesized in vaccinia virus-infected cells. J. Gen. Virol. 78:3297-3302. - PubMed
-
- Belyakov, I. M., L. S. Wyatt, J. D. Ahlers, P. Earl, D. Pendleton, B. L. Kelsall, W. Strober, B. Moss, and J. A. Berzofsky. 1998. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J. Virol. 72:8264-8272. - PMC - PubMed
-
- Carroll, M. W., and B. Moss. 1997. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 238:198-211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

