Critical role for protein tyrosine phosphatase SHP-1 in controlling infection of central nervous system glia and demyelination by Theiler's murine encephalomyelitis virus
- PMID: 12134038
- PMCID: PMC155140
- DOI: 10.1128/jvi.76.16.8335-8346.2002
Critical role for protein tyrosine phosphatase SHP-1 in controlling infection of central nervous system glia and demyelination by Theiler's murine encephalomyelitis virus
Abstract
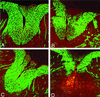
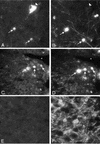
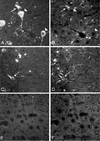
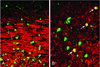
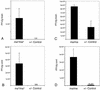
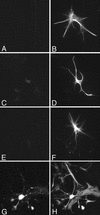
We previously characterized the expression and function of the protein tyrosine phosphatase SHP-1 in the glia of the central nervous system (CNS). In the present study, we describe the role of SHP-1 in virus infection of glia and virus-induced demyelination in the CNS. For in vivo studies, SHP-1-deficient mice and their normal littermates received an intracerebral inoculation of an attenuated strain of Theiler's murine encephalomyelitis virus (TMEV). At various times after infection, virus replication, TMEV antigen expression, and demyelination were monitored. It was found that the CNS of SHP-1-deficient mice uniquely displayed demyelination and contained substantially higher levels of virus than did that of normal littermate mice. Many infected astrocytes and oligodendrocytes were detected in both brains and spinal cords of SHP-1-deficient but not normal littermate mice, showing that the virus replicated and spread at a much higher rate in the glia of SHP-1-deficient animals. To ascertain whether the lack of SHP-1 in the glia was primarily responsible for these differences, glial samples from these mice were cultured in vitro and infected with TMEV. As in vivo, infected astrocytes and oligodendrocytes of SHP-1-deficient mice were much more numerous and produced more virus than did those of normal littermate mice. These findings indicate that SHP-1 is a critical factor in controlling virus replication in the CNS glia and virus-induced demyelination.
Figures



Similar articles
-
Central neuroinvasion and demyelination by inflammatory macrophages after peripheral virus infection is controlled by SHP-1.Viral Immunol. 2009 Dec;22(6):371-87. doi: 10.1089/vim.2009.0052. Viral Immunol. 2009. PMID: 19951174 Free PMC article.
-
Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler's murine encephalomyelitis virus infection leading to demyelination.J Neuroimmunol. 2001 Aug 30;118(2):256-67. doi: 10.1016/s0165-5728(01)00338-1. J Neuroimmunol. 2001. PMID: 11498260
-
Modulation of macrophage infiltration and inflammatory activity by the phosphatase SHP-1 in virus-induced demyelinating disease.J Virol. 2009 Jan;83(2):522-39. doi: 10.1128/JVI.01210-08. Epub 2008 Nov 5. J Virol. 2009. PMID: 18987138 Free PMC article.
-
[Persistentent infection and demyelination induced by Theiler's murine encephalomyelitis virus (TMEV)].Uirusu. 1999 Dec;49(2):175-81. doi: 10.2222/jsv.49.175. Uirusu. 1999. PMID: 10737115 Review. Japanese. No abstract available.
-
Excessive Innate Immunity Steers Pathogenic Adaptive Immunity in the Development of Theiler's Virus-Induced Demyelinating Disease.Int J Mol Sci. 2021 May 17;22(10):5254. doi: 10.3390/ijms22105254. Int J Mol Sci. 2021. PMID: 34067536 Free PMC article. Review.
Cited by
-
Modulation of gene expression in a human cell line caused by poliovirus, vaccinia virus and interferon.Virol J. 2007 Mar 5;4:24. doi: 10.1186/1743-422X-4-24. Virol J. 2007. PMID: 17338811 Free PMC article.
-
Inhibitors of myelination: ECM changes, CSPGs and PTPs.Exp Neurol. 2014 Jan;251:39-46. doi: 10.1016/j.expneurol.2013.10.017. Epub 2013 Nov 4. Exp Neurol. 2014. PMID: 24200549 Free PMC article.
-
Putting on the Brakes: Regulatory Kinases and Phosphatases Maintaining B Cell Anergy.Front Immunol. 2018 Apr 6;9:665. doi: 10.3389/fimmu.2018.00665. eCollection 2018. Front Immunol. 2018. PMID: 29681901 Free PMC article. Review.
-
Central neuroinvasion and demyelination by inflammatory macrophages after peripheral virus infection is controlled by SHP-1.Viral Immunol. 2009 Dec;22(6):371-87. doi: 10.1089/vim.2009.0052. Viral Immunol. 2009. PMID: 19951174 Free PMC article.
-
SHP-1 deficiency and increased inflammatory gene expression in PBMCs of multiple sclerosis patients.Lab Invest. 2008 Mar;88(3):243-55. doi: 10.1038/labinvest.3700720. Epub 2008 Jan 21. Lab Invest. 2008. PMID: 18209728 Free PMC article.
References
-
- Atwood, W. J., L. Wang, L. C. Durham, K. Amemiya, R. G. Traub, and E. O. Major. 1995. Evaluation of the role of cytokine activation in the multiplication of JC virus (JCV) in human fetal glial cells. J. Neurovirol. 1:40-49. - PubMed
-
- Aubert, C., M. Chamorro, and M. Brahic. 1987. Identification of Theiler's virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb. Pathog. 3:319-326. - PubMed
-
- Bansal, R., A. E. Warrington, A. L. Gard, B. Ranscht, and S. E. Pfeiffer. 1989. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J. Neurosci. Res. 24:548-557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

