Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome
- PMID: 12134039
- PMCID: PMC155134
- DOI: 10.1128/jvi.76.16.8347-8359.2002
Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome
Abstract
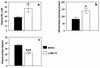
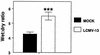
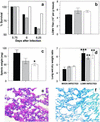
Hantavirus cardiopulmonary syndrome (HCPS) is a life-threatening respiratory disease characterized by profound pulmonary edema and myocardial depression. Most cases of HCPS in North America are caused by Sin Nombre virus (SNV), which is carried asymptomatically by deer mice (Peromyscus maniculatus). The underlying pathophysiology of HCPS is poorly understood. We hypothesized that pathogenic SNV infection results in increased generation of reactive oxygen/nitrogen species (RONS), which contribute to the morbidity and mortality of HCPS. Human disease following infection with SNV or Andes virus was associated with increased nitrotyrosine (NT) adduct formation in the lungs, heart, and plasma and increased expression of inducible nitric oxide synthase (iNOS) in the lungs compared to the results obtained for normal human volunteers. In contrast, NT formation was not increased in the lungs or cardiac tissue from SNV-infected deer mice, even at the time of peak viral antigen expression. In a murine (Mus musculus) model of HCPS (infection of NZB/BLNJ mice with lymphocytic choriomeningitis virus clone 13), HCPS-like disease was associated with elevated expression of iNOS in the lungs and NT formation in plasma, cardiac tissue, and the lungs. In this model, intraperitoneal injection of 1400W, a specific iNOS inhibitor, every 12 h during infection significantly improved survival without affecting intrapulmonary fluid accumulation or viral replication, suggesting that cardiac damage may instead be the cause of mortality. These data indicate that elevated production of RONS is a feature of pathogenic New World hantavirus infection and that pharmacologic blockade of iNOS activity may be of therapeutic benefit in HCPS cases, possibly by ameliorating the myocardial suppressant effects of RONS.
Figures



Similar articles
-
Differential lymphocyte and antibody responses in deer mice infected with Sin Nombre hantavirus or Andes hantavirus.J Virol. 2014 Aug;88(15):8319-31. doi: 10.1128/JVI.00004-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829335 Free PMC article.
-
Development and Characterization of a Sin Nombre Virus Transmission Model in Peromyscus maniculatus.Viruses. 2019 Feb 21;11(2):183. doi: 10.3390/v11020183. Viruses. 2019. PMID: 30795592 Free PMC article.
-
Oral Vaccination With Recombinant Vesicular Stomatitis Virus Expressing Sin Nombre Virus Glycoprotein Prevents Sin Nombre Virus Transmission in Deer Mice.Front Cell Infect Microbiol. 2020 Jul 8;10:333. doi: 10.3389/fcimb.2020.00333. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32733817 Free PMC article.
-
Hantavirus Cardiopulmonary Syndrome in Canada.Emerg Infect Dis. 2020 Dec;26(12):3020-3024. doi: 10.3201/eid2612.202808. Emerg Infect Dis. 2020. PMID: 33219792 Free PMC article. Review.
-
Sin Nombre Virus and the Emergence of Other Hantaviruses: A Review of the Biology, Ecology, and Disease of a Zoonotic Pathogen.Biology (Basel). 2023 Nov 9;12(11):1413. doi: 10.3390/biology12111413. Biology (Basel). 2023. PMID: 37998012 Free PMC article. Review.
Cited by
-
Maporal Hantavirus Causes Mild Pathology in Deer Mice (Peromyscus maniculatus).Viruses. 2016 Oct 18;8(10):286. doi: 10.3390/v8100286. Viruses. 2016. PMID: 27763552 Free PMC article.
-
Seoul virus enhances regulatory and reduces proinflammatory responses in male Norway rats.J Med Virol. 2008 Jul;80(7):1308-18. doi: 10.1002/jmv.21213. J Med Virol. 2008. PMID: 18461618 Free PMC article.
-
Endothelial Nitric Oxide Synthase G894T Polymorphism Associates with Disease Severity in Puumala Hantavirus Infection.PLoS One. 2015 Nov 11;10(11):e0142872. doi: 10.1371/journal.pone.0142872. eCollection 2015. PLoS One. 2015. PMID: 26561052 Free PMC article.
-
Desferrioxamine inhibits protein tyrosine nitration: mechanisms and implications.Free Radic Biol Med. 2012 Aug 15;53(4):951-61. doi: 10.1016/j.freeradbiomed.2012.06.003. Epub 2012 Jun 15. Free Radic Biol Med. 2012. PMID: 22705369 Free PMC article.
-
Inflammation and oxidative stress in vertebrate host-parasite systems.Philos Trans R Soc Lond B Biol Sci. 2009 Jan 12;364(1513):71-83. doi: 10.1098/rstb.2008.0151. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18930878 Free PMC article. Review.
References
-
- Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. - PMC - PubMed
-
- Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. - PubMed
-
- Baluna, R., and E. S. Vitetta. 1997. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology 37:117-132. - PubMed
-
- Beckman, D. L., P. Mehta, V. Hanks, W. H. Rowan, and L. Liu. 2000. Effects of peroxynitrite on pulmonary edema and the oxidative state. Exp. Lung Res. 26:349-359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

