Direct participation of Sam68, the 68-kilodalton Src-associated protein in mitosis, in the CRM1-mediated Rev nuclear export pathway
- PMID: 12134041
- PMCID: PMC155124
- DOI: 10.1128/jvi.76.16.8374-8382.2002
Direct participation of Sam68, the 68-kilodalton Src-associated protein in mitosis, in the CRM1-mediated Rev nuclear export pathway
Abstract
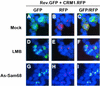
Human immunodeficiency virus type 1 (HIV-1) replication requires efficient nuclear export of incompletely spliced and unspliced HIV-1 mRNA transcripts, which is achieved by Rev expression at an early stage of the viral life cycle. We have recently shown that expression of Sam68, the 68-kDa Src-associated protein in mitosis, is able to alleviate Rev function block in astrocytes by promoting Rev nuclear export. In the present study, we utilized an antisense RNA expression strategy to down-modulate constitutive Sam68 expression and examined its effect on Rev function, HIV-1 gene expression, and viral replication. These results showed that down-modulation of constitutive Sam68 expression markedly inhibited HIV-1 production in 293T cells and viral replication in T lymphocytes such as Jurkat and CEM cells, as well as human peripheral blood mononuclear cells (PBMCs). Rev-dependent in trans complementation and reporter gene assays further demonstrated that inhibition of HIV-1 gene expression by Sam68 down-modulation was due to impeded Rev activity. Moreover, digital fluorescence microscopic imaging revealed that down-modulation of Sam68 expression caused exclusive nuclear retention and colocalization of both Rev and CRM1. Taken together, these data suggest that adequate Sam68 expression is required for Rev function and, thereby, for HIV-1 gene expression and viral replication, and they support the notion that Sam68 is directly involved in the CRM1-mediated Rev nuclear export pathway.
Figures





References
-
- Askjaer, P., T. H. Jensen, J. Nilsson, L. Englmeier, and J. Kjems. 1998. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 273:33414-33422. - PubMed
-
- Bartel, D. P., M. L. Zapp, M. R. Green, and J. W. Szostak. 1991. HIV-1 Rev regulation involves recognition of non-Watson-Crick base pairs in viral RNA. Cell 67:529-536. - PubMed
-
- Battiste, J. L., H. Mao, N. S. Rao, R. Tan, D. R. Muhandiram, L. E. Kay, A. D. Frankel, and J. R. Williamson. 1996. Alpha helix-RNA major groove recognition in an HIV-1 Rev peptide-RRE RNA complex. Science 273:1547-1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

