Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain
- PMID: 12134047
- PMCID: PMC155154
- DOI: 10.1128/jvi.76.16.8446-8454.2002
Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain
Abstract
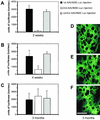
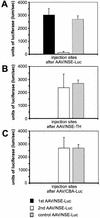
Recombinant adeno-associated viruses (rAAV) are highly efficient vectors for gene delivery into the central nervous system (CNS). However, host inflammatory and immune responses may play a critical role in limiting the use of rAAV vectors for gene therapy and functional genomic studies in vivo. Here, we evaluated the effect of repeated injections of five rAAV vectors expressing different genetic sequences (coding or noncoding) in a range of combinations into the rat brain. Specifically, we wished to determine whether a specific immune or inflammatory response appeared in response to the vector and/or the transgene protein after repeated injections under conditions of mannitol coinjection. We show that readministration of the same rAAV to the CNS is possible if the interval between the first and second injection is more than 4 weeks. Furthermore, our data demonstrate that rAAV vectors carrying different genetic sequences can be administered at intervals of 2 weeks. Our data therefore suggest that the AAV capsid structure is altered by the vector genetic sequence, such that secondary structures of the single-stranded genome have an impact on the antigenicity of the virus. This study provides guidelines for more rational design of gene transfer studies in the rodent brain and, in addition, suggests the use of repeated administration of rAAV as a viable form of therapy for the treatment of chronic diseases.
Figures




References
-
- Anand, V., N. Chirmule, M. Fersh, A. M. Maguire, and J. Bennett. 2000. Additional transduction events after subretinal readministration of recombinant adeno-associated virus. Hum. Gene Ther. 11:449-457. - PubMed
-
- Brockstedt, D. G., G. M. Podsakoff, L. Fong, G. Kurtzman, W. Mueller-Ruchholtz, and E. G. Engleman. 1999. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immunol. 92:67-75. - PubMed
-
- Bromberg, J. S., L. A. Debruyne, and L. Qin. 1998. Interactions between the immune system and gene therapy vectors: bidirectional regulation of response and expression. Adv. Immunol. 69:353-409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

