Differential recognition of tyrosine-based basolateral signals by AP-1B subunit mu1B in polarized epithelial cells
- PMID: 12134076
- PMCID: PMC117320
- DOI: 10.1091/mbc.e01-10-0096
Differential recognition of tyrosine-based basolateral signals by AP-1B subunit mu1B in polarized epithelial cells
Abstract
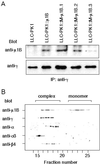
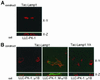
To investigate the importance of tyrosine recognition by the AP-1B clathrin adaptor subunit mu1B for basolateral sorting of integral membrane proteins in polarized epithelial cells, we have produced and characterized a mutant form of mu1B. The mutant (M-mu1B) contains alanine substitutions of each of the four conserved residues, which in the AP-2 adaptor subunit micro2 are critical for interacting with tyrosine-based endocytosis signals. We show M-mu1B is defective for tyrosine binding in vitro, but is nevertheless incorporated into AP-1 complexes in transfected cells. Using LLC-PK1 cells expressing either wild type or M-mu1B, we find that there is inefficient basolateral expression of membrane proteins whose basolateral targeting signals share critical tyrosines with signals for endocytosis. In contrast, membrane proteins whose basolateral targeting signals are distinct from their endocytosis signals (transferrin and low-density lipoprotein receptors) accumulate at the basolateral domain normally, although in a manner that is strictly dependent on mu1B or M-mu1B expression. Our results suggest that mu1B interacts with different classes of basolateral targeting signals in distinct ways.
Figures






References
-
- Aguilar RC, Boehm M, Gorshkova I, Crouch RJ, Tomita K, Saito T, Ohno H, Bonifacino JS. Signal-binding specificity of the {micro}4 subunit of the adaptor protein complex AP-4. J Biol Chem. 2001;276:13145–13152. - PubMed
-
- Aroeti B, Okhrimenko H, Reich V, Orzech E. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim Biophys Acta. 1998;1376:57–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

