Contribution of Ena/VASP proteins to intracellular motility of listeria requires phosphorylation and proline-rich core but not F-actin binding or multimerization
- PMID: 12134077
- PMCID: PMC117321
- DOI: 10.1091/mbc.e02-01-0058
Contribution of Ena/VASP proteins to intracellular motility of listeria requires phosphorylation and proline-rich core but not F-actin binding or multimerization
Abstract
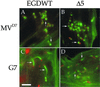
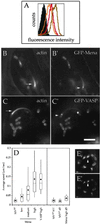
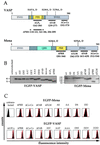
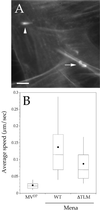
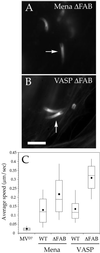
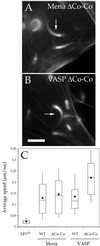
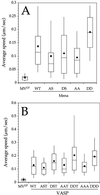
The Listeria model system has been essential for the identification and characterization of key regulators of the actin cytoskeleton such as the Arp2/3 complex and Ena/vasodilator-stimulated phosphoprotein (VASP) proteins. Although the role of Ena/VASP proteins in Listeria motility has been extensively studied, little is known about the contributions of their domains and phosphorylation state to bacterial motility. To address these issues, we have generated a panel of Ena/VASP mutants and, upon expression in Ena/VASP-deficient cells, evaluated their contribution to Ena/VASP function in Listeria motility. The proline-rich region, the putative G-actin binding site, and the Ser/Thr phosphorylation of Ena/VASP proteins are all required for efficient Listeria motility. Surprisingly, the interaction of Ena/VASP proteins with F-actin and their potential ability to form multimers are both dispensable for their involvement in this process. Our data suggest that Ena/VASP proteins contribute to Listeria motility by regulating both the nucleation and elongation of actin filaments at the bacterial surface.
Figures

Similar articles
-
Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phosphoprotein (VASP) function during cell migration.Mol Biol Cell. 2002 Jul;13(7):2533-46. doi: 10.1091/mbc.e01-10-0102. Mol Biol Cell. 2002. PMID: 12134088 Free PMC article.
-
Ena/VASP proteins contribute to Listeria monocytogenes pathogenesis by controlling temporal and spatial persistence of bacterial actin-based motility.Mol Microbiol. 2003 Sep;49(5):1361-75. doi: 10.1046/j.1365-2958.2003.03639.x. Mol Microbiol. 2003. PMID: 12940993
-
Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes.J Cell Biol. 1999 Mar 22;144(6):1245-58. doi: 10.1083/jcb.144.6.1245. J Cell Biol. 1999. PMID: 10087267 Free PMC article.
-
Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration.Annu Rev Cell Dev Biol. 2003;19:541-64. doi: 10.1146/annurev.cellbio.19.050103.103356. Annu Rev Cell Dev Biol. 2003. PMID: 14570581 Review.
-
The focal adhesion phosphoprotein, VASP.Int J Biochem Cell Biol. 1998 Mar;30(3):307-11. doi: 10.1016/s1357-2725(97)00101-5. Int J Biochem Cell Biol. 1998. PMID: 9611773 Review.
Cited by
-
Mena binds α5 integrin directly and modulates α5β1 function.J Cell Biol. 2012 Aug 20;198(4):657-76. doi: 10.1083/jcb.201202079. J Cell Biol. 2012. PMID: 22908313 Free PMC article.
-
Loss of Ena/VASP interferes with lamellipodium architecture, motility and integrin-dependent adhesion.Elife. 2020 May 11;9:e55351. doi: 10.7554/eLife.55351. Elife. 2020. PMID: 32391788 Free PMC article.
-
Loss of profilin-1 expression enhances breast cancer cell motility by Ena/VASP proteins.J Cell Physiol. 2009 May;219(2):354-64. doi: 10.1002/jcp.21677. J Cell Physiol. 2009. PMID: 19115233 Free PMC article.
-
Defining a core set of actin cytoskeletal proteins critical for actin-based motility of Rickettsia.Cell Host Microbe. 2010 May 20;7(5):388-98. doi: 10.1016/j.chom.2010.04.008. Cell Host Microbe. 2010. PMID: 20478540 Free PMC article.
-
Molecular insights on context-specific role of profilin-1 in cell migration.Cell Adh Migr. 2012 Sep-Oct;6(5):442-9. doi: 10.4161/cam.21832. Epub 2012 Sep 1. Cell Adh Migr. 2012. PMID: 23076048 Free PMC article. Review.
References
-
- Ahern-Djamali SM, Comer AR, Bachmann C, Kastenmeier AS, Reddy SK, Beckerle MC, Walter U, Hoffmann FM. Mutations in Drosophila Enabled and rescue by human vasodilator-stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains. Mol Biol Cell. 1998;9:2157–2171. - PMC - PubMed
-
- Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–23557. - PubMed
-
- Bear JE, Loureiro JJ, Libova I, Fassler R, Wehland J, Gertler FB. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. - PubMed
-
- Bear JE, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

