Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins
- PMID: 12134078
- PMCID: PMC117322
- DOI: 10.1091/mbc.e02-01-0021
Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins
Abstract
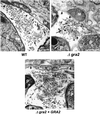
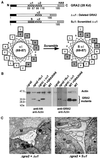
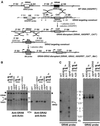
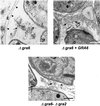
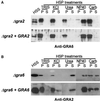
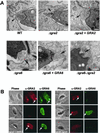
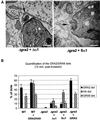
The intracellular parasite Toxoplasma gondii develops within a nonfusogenic vacuole containing a network of elongated nanotubules that form connections with the vacuolar membrane. Parasite secretory proteins discharged from dense granules (known as GRA proteins) decorate this intravacuolar network after invasion. Herein, we show using specific gene knockout mutants, that the unique nanotubule conformation of the network is induced by the parasite secretory protein GRA2 and further stabilized by GRA6. The vacuolar compartment generated by GRA2 knockout parasites was dramatically disorganized, and the normally tubular network was replaced by small aggregated material. The defect observed in Deltagra2 parasites was evident from the initial stages of network formation when a prominent cluster of multilamellar vesicles forms at a posterior invagination of the parasite. The secretory protein GRA6 failed to localize properly to this posterior organizing center in Deltagra2 cells, indicating that this early conformation is essential to proper assembly of the network. Construction of a Deltagra6 mutant also led to an altered mature network characterized by small vesicles instead of elongated nanotubules; however, the initial formation of the posterior organizing center was normal. Complementation of the Deltagra2 knockout with mutated forms of GRA2 showed that the integrity of both amphipathic alpha-helices of the protein is required for correct formation of the network. The induction of nanotubues by the parasite protein GRA2 may be a conserved feature of amphipathic alpha-helical regions, which have also been implicated in the organization of Golgi nanotubules and endocytic vesicles in mammalian cells.
Figures
References
-
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. - PubMed
-
- Cesbron-Delauw MF. Dense granule proteins in Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today. 1994;10:293–296. - PubMed
-
- Charif H, Darcy F, Torpier G, Cesbron-Delauw MF, Capron A. Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp Parasitol. 1990;71:114–124. - PubMed
-
- Dobrowolski JM, INiesman IR, Sibley LD. Actin in the parasite Toxoplasma gondii is encoded by a single copy gene, ACT1 and exists primarily in a globular form. Cell Motil Cytoskeleton. 1997;37:253–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

